INTRODUCTION
Amphibians of Hispaniola comprise 73 species, and 59% of this fauna inhabit the Dominican Republic. The Eleutherodactylus abbotti species group belongs to the subgenus Eleutherodactylus and is represented by seven species (sensu Hedges et al., 2008): E. abbotti, E. audanti, E. haitianus, E. melatrigonum, E. notidodes, E. parabates and E. pituinus. Until the last taxonomic review by Hedges et al. (2008), E. audanti was for a long time considered to be a polytypic species, with one subspecies restricted to each of the main mountain ranges of Hispaniola: E. a. audanti Cochran, 1934 (Massif de la Hotte, Massif de la Selle, Sierra de Bahoruco), E. a. melatrigonum Schwartz, 1966 (Cordillera Central), and E. a. notidodes Schwartz, 1966 (Sierra de Neiba). Eleutherodactylus neodreptus (East side of Sierra de Bahoruco) was described by Schwartz (1965) from a single specimen and synonymized with E. audanti (sensu stricto) by Hedges (1996). Other species morphologically related to E. audanti are E. parabates (Sierra de Neiba) and E. haitianus (Cordillera Central). The taxonomy of the E. abbotti species group is still controversial, since some populations show morphological, genetic and bioacoustic distinctions enough to be considered as different taxa. One of these populations was discovered by one of the authors (MR) not far away from the city of Santo Domingo, on the southeastern slope of the Cordillera Central, showing an intermediate morphology between E. haitianus and E. audanti.
OBJECTIVES
To describe the above mentioned population as a new species.
To provide comparative information on other related taxa of the Eleutherodactylus abbotti species group.
MATERIALS AND METHODS
External morphology. Measurements were taken with digital calipers (accuracy 0.01 mm) under a dissecting microscope Leica MZ-12. Most of the morphological measurements follow Watters et al. (2016), except for the hand length that was taken from the distal border of palmar tubercle to the tip of third finger disc (instead of fourth). This decision was made because in most species finger III is longer than IV (not the opposite condition). We are using tympanum width (horizontally taken like Watters et al., 2016) and also the tympanum height (vertically measured) in our data set for Eleutherodactylus, considering that the shape of this structure is variable among species and the tympanum is not always round enough to be characterized by only one diameter. The diameter of the digital discs was measured if discs looked turgid and not deformed by specimen dehydration.
Molecular phylogeny. DNA was extracted either from liver or leg muscle following the protocol of Ivanova et al. (2006). To eliminate potential PCR-inhibiting contaminants, the tissue samples were incubated for 14 hrs at 4°C in 200 µL low PBS buffer (20 µL PBS in 180µL of water) before overnight digestion with the vertebrate lysis buffer at 56°C. After extraction, the DNA was eluted in 50 µL TE buffer. A fragment of the mitochondrial 16S rRNA gene was amplified in an Eppendorf Mastercycler® pro using the following protocol: initial denaturation for 2 min at 94°C; followed by 40 cycles with denaturation for 35 s at 94°C, hybridization for 35 s at 48.5°C, and elongation for 60 s at 72°C; final elongation for 10 min at 72°C. The reaction mix for each sample contained 1 µL DNA template, 14 µL water, 2.5 µL PCR-buffer, 1 µL 25 mM MgCl2, 4 µL 2.5 mM dNTPs (Invitrogen), 0.5 µL (containing 2.5 units) Taq Polymerase (PeqLab), and 1 µL of each primer (forward: L2510, 5’–CGCCTGTTTATCAAAAACAT–3’; reverse: H3056, 5’–CCGGTCTGAACTCAGATCACGT–3’; Eurofins MWG Operon).
Phylogenetic relationships were estimated using both maximum likelihood (ML) and Bayesian methods. We determined an appropriate model of sequence evolution and model parameters using Kakusan 4.0 (Tanabe, 2011), resulting in the J2 + Gamma model and the GTR + Gamma model for the ML tree and the Bayesian tree, respectively, based on the Akaike information criterion. ML estimation was performed using Treefinder (Jobb et al., 2004) and their robustness was validated using bootstrap analysis with 1000 replications. Bayesian estimation to confirm the ML topology was conducted using MrBayes version 3.1.2 (Ronquist and Huelsenbeck, 2003). We performed 10 million generations of Markov chain Monte Carlo (MCMC) with a sampling frequency of 1000. The MCMC convergence was verified using Tracer 1.7 (Rambaut et al., 2018) and the first 2000 trees were discarded as burn-in, with the remaining samples being used to estimate the tree topology.
Bioacoustic analysis. Calls were recorded with a Marantz PMD 661 digital sound recorder and a Sennheiser ME 66 microphone. Bioacoustic data were obtained with the software Raven Pro 1.5 (Cornell Laboratory of Bioacoustics). Figures were generated with the software BatSound 5.1 (Pettersson Elektronic AB ©1996-1999) using a FFT of 512 points and Hanning windows. Bioacoustical terms and general methods follow Kӧhler et al. (2017). For this group of frogs we are considering that advertisement call is either (1) a train of short notes periodically repeated as a tight unit (as typical of most species) or (2) repetitive longer signals which are well spaced each other along a period of time and not assembled as tight trains (the case of E. parabates).
The following abbreviations are used to refer to institutions and collections: MNHNSD, Museo Nacional de Historia Natural “Prof. Eugenio de Jesús Marcano”, Santo Domingo, República Dominicana; PRRD, field numbers of Proyecto Anfibios Amenazados y Cambio Climático en República Dominicana (specimens deposited at MNHNSD); SMF, Senckenberg, Forschungsinstitut und Naturmuseum, Frankfurt, Germany. Specimens other than type series are listed in Appendix 1. Geographic coordinates are in WGS84 (World Geodetic System 1984).
Eleutherodactylus geitonos sp. nov.
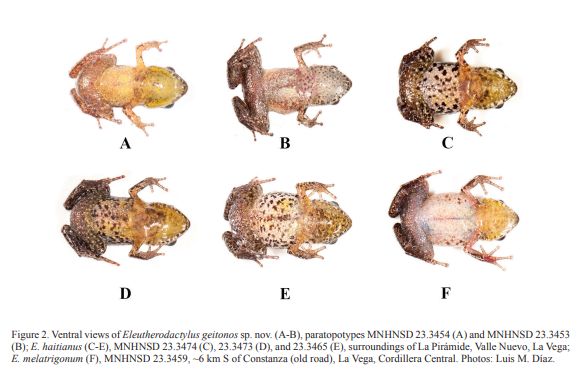
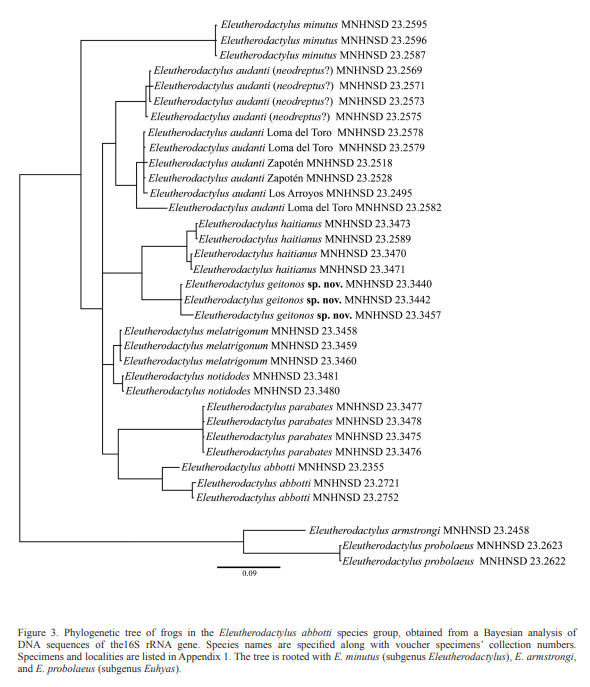
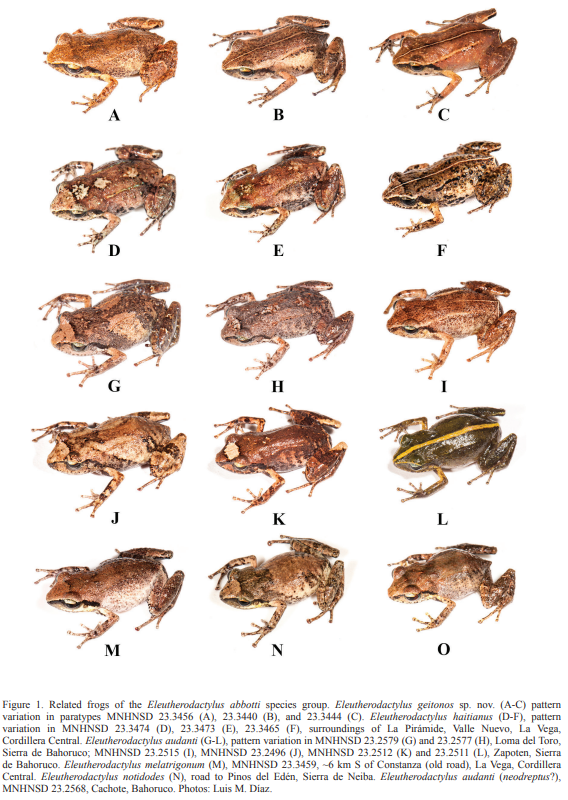
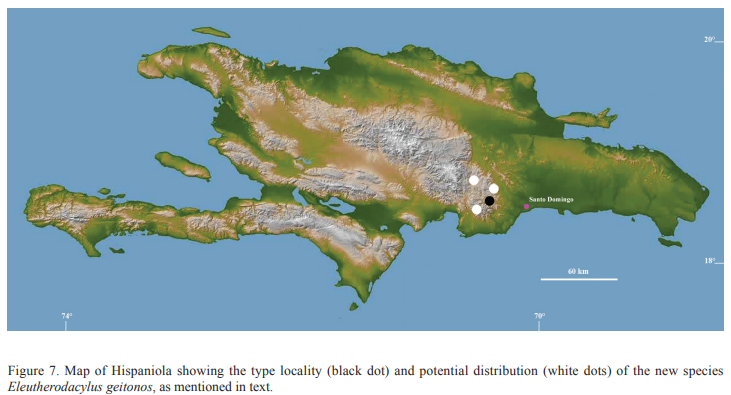
Figures: external morphology, 1A-C and 2A-B; phylogeny, 3; holotype, 4; vomerine odontophores, 5; sonagrams and oscilograms, 6A, G; distribution, 7; habitat, 8.
Holotype. MNHNSD 23.3438 (original field number PRRD 670), adult male from 3.8 km NW of El Corte Nuevo (18°29’17.29”N; 70°16’38.49”W), 1084 m above the sea level, Cambita Garabitos Municipality, San Cristóbal Province, southeastern slope of Cordillera Central, Dominican Republic, collected by Luis M. Díaz, Cristian Marte and Marcos Rodríguez on February 9, 2014.
Paratypes (n=20). Males (n=10): SMF 103894, MNHNSD 23.3443, MNHNSD 23.3448–51, SMF 103895–96, MNHNSD 23.3456–57 (original field numbers PRRD 661, 665, 671–74, 681–82, 683–684, respectively). Females (n=10): SMF 10397–98, MNHNSD 23.3440–42, 23.3444–3447, 23.3453 (original numbers PRRD 642–43, 662–64, 666–69, 680), same collecting data as holotype.
Diagnosis. A small species (maximum SVL in males 12.8 mm, in females 15.8 mm) of the Eleutherodactylus abbotti species group of the E. auriculatus species series (sensu Hedges et al., 2008; confirmed by Padial et al., 2014) as supported by morphological and genetic data (Figs. 1-3). It requires the closest comparison with E. haitianus but also with E. audanti and related species (E. melatrigonum, E. notidodes, and E. parabates; see Discussion).
From those species, E. geitonos sp. nov. differs in lacking a pectoral fold and by having a small vocal sac that is not distinctively folded when deflated. Eleutherodactylus geitonos sp. nov. and E. haitianus are both very small frogs and the former, on average, has an even smaller size than the latter (see Table I). The new species has more distinctive digital discs than E. haitianus (third finger disc 3.1–4.0% of SVL, x̅ =3.4%, vs. 1.8–2.9%, x̅ =2.4%, in E. haitianus); a relatively longer snout (15–17% of SVL, vs. 12–15% in E. haitianus), and a pair of incomplete dorsolateral folds (vs. dorsolateral rows of tubercles in E. haitianus, with very prominent ones at the suprascapular level). Eleutherodactylus geitonos sp. nov. lacks the pattern of dark spots that E. haitianus typically has on belly and throat, and yellow coloration is more extended to ventral surfaces of males than in the latter species (Fig. 2). Advertisement calls of E. geitonos sp. nov. are long trains of notes like in E. haitianus (Fig. 6), but in the latter species’ call, the introductory note is long, somewhat frequency modulated, while a distinctive call introductory note is not present in E. geitonos sp. nov. From E. audanti and closely related taxa, the new species also differs in being much smaller (see Table I); in E. audanti toe V is longer than toe III, but in the new species these toes are of similar size or III>V. Eleutherodactylus parabates is also a larger species (up to 24 mm SVL; Schwartz and Henderson, 1991) with stocky body and advertisement calls consisting of long whistles.
Diagnosis (Español). Especie de pequeño tamaño (LHC máxima en los machos, 12.8 mm; hembras, 15.8 mm) del grupo de especies E. abbotti, de la serie E. auriculatus (sensu Hedges et al, 2008; confirmado por Padial et al., 2014), como sugieren los datos morfológicos y genéticos (Figs. 1-3). Requiere una estrecha comparación con E. haitianus, pero también con E. audanti y las especies afines a ésta (E. melatrigonum, E. notidodes, y E. parabates; véase Discusión). De todas estas especies E. geitonos sp. nov. se diferencia por la carencia de pliegue pectoral y por tener un saco vocal pequeño que no se pliega apreciablemente cuando está desinflado. Tanto Eleutherodactylus geitonos sp. nov. como E. haitianus son ranas muy pequeñas y el nuevo taxón tiene una talla promedio aún más reducida que la segunda especie (Tabla I). La nueva especie tiene los discos digitales de las manos más desarrollados que E. haitianus (tercer dedo 3.1–4.0% de la LHC, x̅=3.4%, vs. 1.8–2.9%, x̅=2.4%, en E. haitianus); un hocico relativamente más largo (15–17% de la LHC vs. 12–15%, en E. haitianus); un par de pliegues dorsolaterales incompletos (vs. hileras dorsolaterales de tubérculos en E. haitianus, siendo los de la región supraescapular muy prominentes). Eleutherodactylus geitonos sp. nov. no tiene un manchado conspicuo en la garganta y el vientre como típicamente ocurre en Eleutherodactylus haitianus, y la coloración amarilla está más extendida sobre las superficies ventrales de los machos que en la segunda especie (Fig. 2). Las llamadas de anuncio de E. geitonos sp. nov. son largos trenes de notas como en E. haitianus (Fig. 5), pero en la última especie la nota introductoria de cada llamada es larga y con cierta modulación de frecuencia, mientras que no existe una nota introductoria diferenciada de esta manera en E. geitonos sp. nov. De E. audanti y taxones cercanos, la especie nueva se diferencia por ser mucho más pequeña (Tabla I); en E. audanti el dedo V del pie es más largo que el III, pero en la nueva especie estos dedos tienen una longitud similar ó III>V. Eleutherodactylus parabates es una especie de mayor tamaño (hasta 24 mm LHC; Schwartz and Henderson, 1991), con un cuerpo robusto y llamadas de anuncio consistentes en prolongados silbidos.

Description. Head as wide as long, head width 90–100% (x̅ =97%) of head length; snout subacuminate in dorsal view and in profile, slightly overlapping the lower jaw; snout length 38–44% (x̅ =41%) of head length; nostrils oval, weakly protuberant, directed laterally, and separated by a distance equivalent to 26–32% (x̅ =29%) of head width; canthus rostralis straight in dorsal view and rounded in profile; loreal region gradually sloping to the labial border; lips not flared; interorbital distance 32–39% (x̅ =36%) of the head width; upper eyelid 48–66% (x̅ =58%) of the interorbital distance; eyelid skin with very small granules; loreal area smooth; tympanum small, superficial, rounded, with distinct annulus, 25–50% (x̅ =37%) of eye diameter; supratympanic fold conspicuously pigmented with black; 1 to 3 postrictal tubercles aligned to form a light area below the posterior half of the supratympanic fold; choanae 51–63% (x̅ =58%) of the third finger disc diameter, oval, lateral in position, partially hidden by the palatal shelf of the maxillary arch; vomerine odontophores very small, bearing 7 to 8 teeth, 1¼ times the length of each choana, situated in a diagonal position between the choanae and separated from each other by a distance equivalent to half their own length (Fig. 5); tongue oval, 3/4 not adherent to floor of mouth; external vocal sac of males subgular, small, round shaped, and not distinctively folded when deflated; vocal slits present.
Dorsal skin with scattered granules, and fully covered with glandular pores;
dorsolateral tubercles aligned and fused to form incomplete folds; lower surface of
flanks areolate. A well evident supratympanic gland, but other glands not conspicuously
swollen. Venter areolate. Inner surface of thigh
areolate. Palmar tubercle rounded to oval, smooth, 1¾ times longer than thenar tubercle;
supernumerary palmar tubercles absent; subarticular tubercles of fingers rounded and
moderately prominent. Finger length order: III > IV > II > I; digital discs moderately enlarged and somewhat
expanded laterally, the disc on finger III 1.5-1.6 times wider than the phalange width
at base and its diameter is 51–85% (x̅ =62%) of tympanum width; discs are larger in the
two outer fingers but slightly decrease in size on
disc II and I. Heels without enlarged tubercles;
inner metatarsal tubercle two times longer than the slightly conical outer metatarsal
tubercle; supernumerary tubercles absent; subarticular tubercles rounded to oval and
moderately prominent in profile. Toes without defined
lateral ridges or basal webbing; circumferential
groove bordering the distal half of toe pad; heels separated or barely touching each
other when flexed legs are held at right angles to sagittal plane; toes length order: IV> V ≥ III > II > I. Hand length 20–25% (x̅
=23%) of SVL; foot length 38–44% (x̅ =42%) of SVL; thigh length 38–48% (x̅ =43%) of SVL;
shank length 42–51% (x̅ =46%) of SVL; tarsal length 28–39% (x̅ =31%) of SVL.
Measurements are summarized in Table I. Color in alcohol of the holotype
(three years after collection): dorsum brownish-gray with a dark chevron-like
suprascapular pattern (Fig. 4). Yellow tonalities were not preserved. Some whitish
tubercles are defined in the suprascapular area. Interocular bar very evident. A white sagittal hairline is extended along
middorsum to the vent, where it bifurcates onto the inner surface of hind limbs and
plantar surface. A similar line is also present on inner surfaces of forelimbs and
hands. Inner surface of fore- and hind limbs,
distinctively darken. Snout, anterior to interocular bar, paler than dorsum but darker
at both sides of the sagittal hairline. Snout, in dorsal view, and the external edge of
eyelids with a whitish outline. Thigh, shank, and tarsus, crossed by wide, pale outlined dark bands. Vent area and proximal thigh
inner surface defining a dark triangle, outlined above by the bifurcations of the
sagittal hairline. Forelimbs with two incomplete antebrachial dark bars. Arms
distinctively paler than dorsum. Dorsum somewhat
stippled. The dorsolateral folds slightly paler than middorsum. Ventral surface,
including throat, belly and hind legs, finely stippled on a white background. Chest with
some dark spots. Mandibles with dark blotches. Lores crossed by a dark stripe from the tip of snout to eye. Supratympanic fold
black, extended to forelimbs; area under this fold and behind tympanum distinctively
paler. Color in life: dorsum brownish
tan, brown to reddish brown, with variably highlighted yellowish tones on arms. Hind limbs with broad brown bands outlined with a paler
tonality. Supratympanic fold very dark, almost black, extended to forelimbs. Area below
the supratympnic fold and behind tympanum usually yellow. Lores crossed by a dark brown
stripe, which is continuous with the dark lower-half
of the iris. Eleutherodactylus geitonos sp. nov., is a polymorphic species with different dorsal
patterns. A dorsal, chevron-like brown figure is more or less defined at the level of
forelegs in many individuals (including the
holotype). An interocular bar is usually
present, often with a brown extension onto the suprascapular chevron (Fig. 1A). Snout,
anterior to interocular bar, slightly paler than dorsum. Flanks with the same color as
dorsum or with a broad and distinctive cream area
(Fig. 1B). Some individuals with narrow, dorsolateral orange stripes (Fig. 1C). A
whitish to tan middorsal hairline is evident in most individuals. Belly and throat
typically yellow in males, but grayish in young individuals and in females. Advertisement calls and bioacoustics comparison. Eleutherodactylus geitonos sp. nov. and E. haitianus are the two
species in the E.
abbotti species group with the highest note-
repetition rate and, correspondingly, the shortest note periods. Also, both species have the highest pitched calls as expected by their
smaller sizes. Table II compares the call parameters of E. geitonos sp. nov. with E. haitianus, but also with those of E. audanti and closely
related species that share an advertisement call pattern consisting of a train of notes.
Advertisement calls of
Eleutherodactylus
geitonos sp. nov. have 13–44 notes (x̅ =26.1). Notes
are clicks that increase in intensity gradually within each call. Calls usually end abruptly (Fig. 6G). Call period is very
variable, 14–75.8 (x̅ =33.1) seconds; note duration, 11–29 (x̅ =17.4) milliseconds; note
period, 124–183 (x̅ =146) milliseconds; notes rise time, 1–4 (x̅ =1.8) milliseconds;
notes repetition rate 6.2–9.1 (x̅ =7.1) notes/second.
The dominant frequency is 5.0–5.6 (x̅ =5.3) kHz. Note´s frequency is somewhat modulated
or have no modulation. In E. haitianus, the calls
usually start with a distinctive note that is 68–115 (x̅ =90) milliseconds long,
followed by shorter signals of 12–28 (x̅ =15)
milliseconds. First note duration is not distinctively differentiated in E. geitonos sp. nov. as shown in Table II, and
has the lowest intensity of the call (Fig. 6G). Typically, notes of E. haitianus gradually decrease intensity
at the end of each call (Fig. 6H). Eleutherodactylus melatrigonum and E. notidodes emit the
longest calls of the group, with some of them almost lasting one minute (Table II). In
E. notidodes there were few calls in the upper limit of E. audanti, barely
overlapping in call duration. These two species also showed lower dominant frequencies
than E.
audanti, very likely due to their somewhat larger
sizes and maybe the influence of some environmental factors. The only measured
E. melatrigonum
has shorter notes than E.
notidodes. Eleutherodactylus parabates produces long, single noted whistles (Fig. 6F) instead of the long
trains of clicking notes of the other species. This species is sympatric with
E. notidodes and an as-yet undescribed species related to E. audanti. They all
vocalize in the same area but inhabiting different microhabitats. Eleutherodactylus
parabates is the only species that wasn´t included in
Table II due to its very different call pattern. We only had one male available
for measurements, but many were heard and the
whistling nature of vocalizations was referred by Schwartz (1966) in the species’
original description. Calls have a duration of 215–267 (x̅ =233; n=15) milliseconds,
call period: 411–461(x̅ =433; n=14) milliseconds,
dominant frequency: 2.6–2.8 (x̅ =2.7 kHz; n=15) kHz, and a repetition rate of 141
calls/minute. Calls rise time: 130–180 (x̅ =149, n=15) milliseconds. Each call has a
slight ascendant modulated period in the first 20–30 milliseconds of its
duration. Distribution. The new
species is only known from the type locality (Fig. 7). However, frogs with similar call
patterns and habitats have been recorded at Rancho Arriba (Sierra de Ocoa; San José de
Ocoa Province), El Valle de Dios (Parque Nacional Loma La Humeadora; San Cristobal Province), and Los Guayuyos (near Parque Nacional Luis
Quin; Peravia Province). Additional surveys will confirm the geographic distribution of
the new species in those potential localities and nearby areas. Etymology. The specific
epithet is from the ancient Greek γείτων, geitȏn, meaning a
neighbor, in allusion to the proximity of the type locality to the city of Santo
Domingo.
Ecological notes. At the type locality (Fig. 8), the habitat of E. geitonos sp. nov. is a mesophilous cloud forest (“bosque latifoliado nubladoˮ; as described by Hager and Zanoni, 1993:
60), with patches of palm forests (Prestoea montana) locally
known as “manaclaresˮ. The area has been modified by human activities along with some
deforestation, cattle farming and agriculture. The
new species was heard calling either inside the forest or along trail-sides covered by
dense vegetation. The new frog is primarily associated with the grass stratum. Males
start vocalizing activity at dusk and the type series was collected after 19:00 hours, either on plants or on the ground in an area of less
than 600 m2. About
twenty calling males were detected there. Temperature was 16°C and the relative humidity
94%. Calling males were either found on the ground or, very often, perched on
ferns. Males were hidden in fern´s trunk crevices,
protected inside dead fern fronds, or among the leaves of other nearby plants. Calling
sites were less than one meter high. Females were found on the leaf litter. Other
species of Eleutherodactylus
recorded with E. geitonos sp. nov. were
E. abbotti, E. auriculatoides, and
E. inoptatus.
DISCUSSION
The obtained phylogeny (Fig. 3) agree with the general results of Hedges et al. (2008) and Padial et al. (2014). It confirms the sister relationships of E. geitonos sp. nov. with E. haitianus and its inclusion in the E. abbotti species group. The tree also show that the sampled population of E. audanti from Cachote, Eastern side of Sierra de Bahoruco, is genetically distinct from those of the western side (Loma del Toro, Zapotén, and Los Arroyos), which might indicate that E. neodreptus should be resurrected. To complicate this issue even more, Schwartz and Henderson (1991) mapped, and Hedges (1996) later discussed that the distribution of E. audanti extends to the East of Bahoruco reaching the same area of E. neodreptus. This is a new field of research since E. neodreptus was described from only one specimen. As such, additional morphological, genetic and bioacoustic comparisons using a larger set of data from multiple localities have to be conducted to clarify the taxonomic status of this controversial group of cryptic frogs.
Our genetic data barely separate E. notidodes and E. melatrigonum; the two species probably represent a single taxon (E. notidodes) that has a wide distribution in the north paleoisland. Although Hedges et al. (2008) elevated the two taxa to species level, only E. notidodes was included in their phylogeny and the taxonomic status of E. melatrigonum was inferred based on described morphology (Schwartz, 1966) and its allopatric distribution. Padial et al. (2014) did not include E. notidodes and E. melatrigonum in their analysis, but recognized them as different species within the E. abbotti species group. We are aware that our phylogeny is limited to the 16S rRNA gene, and thus constitutes a matrilineal genealogy only; however, this gene has proven to be very useful for species delimitations in amphibians (ie: Vences et al., 2005; Vences et al., 2008; Maya-Soriano et al., 2012; Grosjean et al., 2015; Rockney et al., 2015; Ohler and Nicolas, 2017).
Eleutherodactylus geitonos sp. nov. is readily distinguished
from E.
abbotti, a sympatric but larger species (up to 25 mm SVL; Schwartz and Henderson,
1991) that lacks defined dorsolateral folds, has advertisement calls (described by
Galvis et al. 2016) consisting of
heterogeneous notes which are rhythmically uttered in complex assemblages, the body exhibits different color patterns and the genetic
relationship with the new species is more distant (as shown in Fig. 3). Eleutherodactylus pituinus
is a completely different species that do not offer
confusion with the new species; it reach 29 mm SVL
(Schwartz and Henderson, 1991), lack dorsolateral folds, has dark brown concealed
surfaces on legs, a prominently stippling dorsal pattern, more conspicuous subarticular
tubercles, and very distinctive advertisement calls. Ecological differences
also distinguish E. geitonos sp. nov. and
E. haitianus. In contrast with the new species, E. haitianus typically occurs in high elevation valleys (usually ≥2000 m
a.s.l.) having highland pine forests (Pinus occidentalis) and
the edges of mesophilous forests (see descriptions by
Hager and Zanoni, 1993:69). Frogs are usually associated to the grass stratum, particularly in those habitats dominated by the grass
Danthonia
domingensis (Poaceae), which is locally known as
pajón. Males call either hidden in these plants or they climb to get exposed among the thin leaves, although
they can be found calling from other grasses, low bushes and ferns (Henderson and
Powell, 2009).
ACKNOWLEDGMENT
We are grateful to Nils Navarro, Kenia Ng, Eveling Gavot and Miguel A. Landestoy for their assistance during field work. Many thanks to Linda Mogk (Senckenberg, Forschungsinstitut und Naturmuseum Frankfurt, Germany), for obtaining the genetic data and her kind assistance in the lab. Arne Schulze helped us with specimen photographs. We acknowledge Celeste Mir and Carlos Suriel (Museo de Historia Natural of Santo Domingo) for their kind support and the work space at the Museum. This paper is an outcome of the project Anfibios Amenazados y Cambio Climático en República Dominicana (Endangered Amphibians and Climate Change in Dominican Republic), financed by Ministerio de Educación Superior, Ciencia y Tecnología, FONDOCYT 2008-1-A-102, of Dominican Republic, and its conclusion was also supported by the project Taxonomía de algunos grupos zoológicos de Cuba y del Caribe, con acciones de capacitación especializada, divulgación, y educación ambiental (Museo Nacional de Historia Natural de Cuba). Our gratitude to Grupo Jaragua (Santo Domingo) for the administrative and logistic support of the Project, with especial thanks to Yvonne Arias, Yolanda León, and Miguel Abreu. Also to the authorities of Museo Nacional de Historia Natural de Cuba, Agencia de Medio Ambiente (CITMA, Cuba), and Sociedad Cubana de Zoología for supporting the Cuban participants of the Project. LMD wishes to express his gratitude to the University of Würzburg (particularly to Michael Schmid, Wolfgang Feichtingeri, and Claus Steinlein), and the Belgian Focal Point to the Global Taxonomy Initiative (especially to Yves Samyn) for providing the photographic equipment. Sound recording equipment and Raven Pro was kindly donated by the Cornell Lab of Ornithology, and we especially wish to thank Greg Budney, John Fitzpatrick, and Eduardo Iñigo for their extraordinary support over several years. Thanks to Kraig Adler, Martha Muñoz, Robert Murphy and Shannan Yates, for their very welcomed comments and suggestions on different versions of manuscript.
LITERATURA CITADA
Galvis, P. A., V. Zaffaroni, S. J. Sánchez-Pacheco and M. Rada. 2016. The advertisement calls of three Eleutherodactylus species from Hispaniola (Anura: Eleutherodactylidae). Bioacoustics, DOI: 10.1080/09524622.2016.1260053.
Grosjean, S., A. Ohler, Y. Chuaynkern, C. Cruaud and A. Hassanin. 2015. Improving biodiversity assessment of anuran amphibians using DNA barcoding of tadpoles. Case studies from Southeast Asia. Comptes Rendus Biologies, 338: 351–361.
Hager, J. and T. A. Zanoni. 1993. La vegetación natural de la República Dominicana: una nueva clasificación. Moscosoa, 7: 39–81.
Hedges, S. B. 1996. The Hispaniolan frog Eleutherodactylus neodreptus Schwartz (Anura: Leptodactylidae) is a synonym of E. audanti Cochran. Caribbean Journal of Science, 32: 248.
Hedges, S. B., W. E. Duellman, and M.P. Heinicke. 2008. New World direct developing frogs (Anura: Terrarana): Molecular phylogeny, classification, biogeography, and conservation. Zootaxa, 1737: 1–182.
Henderson, R. W., and R. Powell. 2009. Natural History of West Indian reptiles and amphibians. University Press of Florida, 493 pp.
Ivanova, N. V., J. De Waard and P. D. N. Hebert. 2006. An inexpensive, automation-friendly protocol for recovering high-quality DNA. Molecular Ecology Notes, 6: 998–1002.
Jobb, G., A. von Haeseler, and K. Strimmer. 2004. TREEFINDER: a powerful graphical analysis environment for molecular phylogenetics. BMC Evolutionary Biology, 4:18.
Köhler, J., M. Jansen, A. Rodríguez, P. J. R. Kok , L. F. Toledo , M. Emmrich, F. Glaw, C. F. B. Haddad, M. O. Rödel, and M. Vences. 2017. The use of bioacoustics in anuran taxonomy: theory, terminology, methods and recommendations for best practice. Zootaxa, 4251(1): 1–124. doi: 10.11646/zootaxa.4251.1.1.
Maya-Soriano, M. J., W. V. Holt and R. E. Lloyd. 2012. Biobanked amphibian samples confirmed to species level using 16S rRNA DNA barcodes. Biopreserv. biobank, 10: 22–28.
Ohler, A. and V. Nicolas. 2017. Which frog’s legs do froggies eat? The use of DNA barcoding for identification of deep frozen frog legs (Dicroglossidae, Amphibia) commercialized in France. European Journal of Taxonomy, 271: 1–19.
Padial, J. M., T. Grant and D. R. Frost. 2014. Molecular systematics of terraranas (Anura: Brachycephaloidea) with an assessment of the effects of alignment and optimality criteria. Zootaxa, 3825 (1): 001–132.
Rambaut A., A. J. Drummond, D. Xie, G. Baele and M. A. Suchard. 2018. Tracer v1.7, Available from http://beast.community/tracer.
Rockney, H. J., C. Ofori-Boateng, N. Porcino and A. D. Leaché. 2015. A comparison of DNA barcoding markers in West African frogs. African Journal of Herpetology, 64 (2): 135–147.
Ronquist, F. and J. P. Huelsenbeck. 2003. Mrbayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics, 19: 1572–1574.
Schwartz, A. 1965. A new species of Eleutherodactylus (Amphibia: Leptodactylidae) from the Sierra de Baoruco, Dominican Republic. Proceedings Biological Society of Washington, 78: 165–168.
Schwartz, A. 1966. The relationships of four small Hispaniolan Eleutherodactylus (Leptodactylidae). Bulletin Museum of Comparative Zoology, 133: 369–399.
Schwartz, A. and R. W. Henderson. 1991. Amphibians and Reptiles of the West Indies. University of Florida Press, Gainesville, 720 pp.
Tanabe, A. S. 2011. Kakusan4 and Aminosan: two programs for comparing nonpartitioned, proportional and separate models for combined molecular phylogenetic analyses of multilocus sequence data. Molecular Ecology Notes, 11: 914–921.
Vences, M., M. Thomas, A. van der Meijden, Y. Chiari and D. R. Vieites. 2005. Comparative performance of the 16S rRNA gene in DNA barcoding of amphibians. Frontiers in Zoology, 2005, 2:5 doi:10.1186/1742-9994-2-5.
Vences, M., Y. Chiari, M. Teschke, R-D. Randrianiaina, L. Raharivololoniaina, P. Bora, D. R. Vieites and F. Glaw. 2008. Which frogs are out there? A preliminary evaluation of survey techniques and identification reliability of Malagasy amphibian. A Conservation Strategy for the Amphibians of Madagascar. Monografie del Museo Regionale di Scienze Naturali di Torino, XLV (2008): 233–252.
Watters, J. L., S. T. Cummings, R. L. Flanagan and C. D. Siler. 2016. Review of morphometric measurements used in anuran species descriptions and recommendations for a standardized approach. Zootaxa, 4072 (4): 477–495.
APPENDIX I SPECIMENS EXAMINED FOR COMPARISONS AND GENETIC PHYLOGENY
Eleutherodactylus abbotti (n=3).— MNHNSD 23.2721, 23.2752, Aguas Negras, Bahoruco; 23.2355, Cachote, Bahoruco.
Eleutherodactylus armstrongi (n=1).— MNHNSD 23.2458, Polo, Sierra de Bahoruco, Barahona.
Eleutherodactylus audanti (n=93).— MNHNSD 23.2480–2494, 23.2496–2549, Zapoten, Bahoruco. MNHNSD 23.2550–64, 23.2577–2582, Loma del Toro, Bahoruco. MNHNSD 23.2565–67, Las Abejas, Bahoruco.
Eleutherodactylus audanti (neodreptus?, n=9).— MNHNSD 23.2568–76, Cachote, Sierra de Bahoruco, Bahoruco.
Eleutherodactylus haitianus (n=13).— MNHNSD 23.3464–74, Valle Nuevo, Cordillera Central, La Vega Province; MNHNSD 23.3482–83, Parque Nacional José del Carmen Ramírez, San Juan de la Maguana.
Eleutherodactylus melatrigonum (n=6).— MNHNSD 23.3458–63, ~6 km S of Constanza, La Vega.
Eleutherodactylus minutus (n=15).— MNHNSD 23.2585–97, Valle Nuevo, Cordillera Central, La Vega Province; MNHNSD 23.2607–08, Idem; MNHNSD 23.2598–99, Casabito, Ébano Verde, La Vega.
Eleutherodactylus notidodes (n=8).— MNHNSD 23.2600–2606, 23.3480–81, Sabana del Silencio, Sierra de Neiba, Bahoruco Province.
Eleutherodactylus parabates (n=6).— MNHNSD 23.3475–79, Sabana del Silencio, Sierra de Neiba, Bahoruco.
Eleutherodactylus pituinus (n=1).— MNHNSD 23.2584, ~6 km S of Constanza, La Vega.
[Recibido: 30 de abril, 2018. Aceptado para publicación: 14 de junio, 2018]