![]() Número 23,
enero, 2024: 51–73
Número 23,
enero, 2024: 51–73
ISSN versión impresa: 2071–9841 ISSN versión en línea: 2079–0139 https://doi.org/10.33800/nc.vi23.347
DESCRIBING THE DYNAMICS OF RECRUITS AND JUVENILE
SCLERACTINIAN CORALS USING 3D MODELS: A CASE STUDY FROM
CAYO MERO REEF, MORROCOY NATIONAL PARK, VENEZUELA
Descripción de las dinámicas de reclutas y juveniles de corales escleractínidos utilizando modelos 3D: un caso de estudio del arrecife de Cayo Mero, Parque Nacional Morrocoy, Venezuela
Gloria Mariño-Briceño1a*, José Cappelletto2, Alfredo Ascanio3, Esteban Agudo-Adriani4, and Aldo Cróquer1b, 5
1 Laboratorio de Ecología Experimental, Departamento de Estudios Ambientales, Universidad Simón Bolívar, Caracas 1080, Venezuela; a https://orcid.org/0000-0002-8877-3547; b https://orcid.org/0000-0001-8880-9338, aldo.croquer@tnc.org. 2 Grupo de I+D en Mecatrónica, Universidad Simón Bolívar, Caracas 1080, Venezuela; https://orcid.org/0000-0002-8891-6915, cappelletto@usb.ve. 3 Department of Biology, Miami University, Oxford,
Ohio 45056, USA; https://orcid.org/0000-0001-9987-7977, ascanio.alfredoa@gmail.com. 4 Department of Biology, University of North Carolina at Chapel Hill, NC 27599, USA; https://orcid.org/0000-0001-7815-4120, eagudo@unc.live.edu. 5 The Nature Conservancy, Caribbean Division, Central Caribbean Program, Santo Domingo, Dominican Republic. *Corresponding author: gloritam94@gmail.com.
[Received: November 08, 2023. Accepted: January 11, 2024]
ABSTRACT
Understanding the dynamics of coral recruitment and post-settlement is fundamental to a better comprehension of coral reef dynamics and recovery. We studied the abundance and survivorship of coral recruits and juveniles together with benthic dynamics at a scale of months and centimeters in Playa Mero reef, a disturbed reef in Morrocoy National Park. For this, we used photogrammetry to monitor eight permanent 50x50 cm quadrats haphazardly deployed every 3–4 months over 18 months. Juveniles and recruits of Agaricia spp. were at least four times more abundant than reef builders such as Orbicella spp. A distance-based linear model showed that rugosity, macroalgae, coral cover, and sand were the most important benthic variables and predicted up to 46% of the spatial and temporal variation of recruit and juvenile corals. The mortality of juvenile corals was higher than net recruitment rates, and only a limited number of genera such as Agariciids, Colpophyllia, Porites, and Scolymia were observed as recruits. Using a logit model, we also found a positive relationship between the mean growth rate and survivorship of juvenile corals (Nagelkerke R2= 0.67). We concluded the lack of recruitment of large reef builders, and the rapid mortality of a limited number of juvenile species, might be a sign of a coral community's failure to increase coral cover.
Keywords: juvenile corals, disturbed reefs, photogrammetry, structural complexity, growth rates, Caribbean.
RESUMEN
Comprender la dinámica de reclutamiento de corales y la etapa de asentamiento es fundamental para un mejor entendimiento de la dinámica y recuperación de los arrecifes de coral. En este experimento, estudiamos la abundancia y supervivencia de reclutas y juveniles de coral junto con la dinámica bentónica a una escala de meses y centímetros en el arrecife de Playa Mero, un arrecife perturbado en el Parque Nacional Morrocoy. Para esto, utilizamos fotogrametría para monitorear ocho cuadrantes permanentes de 50x50 cm desplegados aleatoriamente cada 3–4 meses durante un período de 18 meses. Los juveniles y reclutas de Agaricia spp. fueron al menos cuatro veces más abundantes que los de constructores de arrecifes como Orbicella spp. Un modelo lineal basado en la distancia mostró que la rugosidad, las macroalgas, la cobertura de coral y la arena eran las variables bentónicas más importantes y predijo hasta un 46% de la variación espacial y temporal de los corales jóvenes y reclutas. La mortalidad de los corales jóvenes fue mayor que las tasas netas de reclutamiento y solo se observó la presencia limitada de géneros como Agarícidos, Colpophyllias, Porites y Scolymias. Usando un modelo logístico, también encontramos una relación positiva entre la tasa de crecimiento promedio y la supervivencia de corales juveniles (Nagelkerke R2 = 0.67). Concluimos que la falta de reclutamiento de grandes constructores de arrecifes y la mortalidad rápida de un número limitado de especies podrían ser señales de una comunidad de coral fallando en la lucha por recuperar la cobertura de coral y, por lo tanto, podría haber ocurrido un cambio de fase en el arrecife estudiado.
Palabras clave: corales juveniles, arrecifes perturbados, fotogrametría, complejidad estructural, tasas de crecimiento, Caribe.
INTRODUCTION
Coral reefs are highly diverse and often resilient ecosystems and their ability to recover from disturbances is a common feature of their evolutionary history (Connell et al., 1997; Mumby & Steneck, 2011). Nevertheless, in the most recent few decades, many reefs have lost live coral cover and are undergoing rapid phase shifts from coral to macroalgal communities from local to global scales (Hughes, 1994; Souter et al., 2021). Understanding the ecological dynamics of highly disturbed reefs can shed light upon the causes of phase shifts, successful recovery, and/ or lack thereof.
Ecological processes acting during the early life stages of corals (i.e., recruitment and post-settlement survivorship/mortality) are important bottlenecks that determine much of coral demography and the fate of coral communities after a disturbance (Birkeland et al., 1981; Chong-Seng et al., 2014; Connell et al., 1997; Edmunds et al., 2015; Hughes et al., 2010; RitsonWilliams et al., 2009; Vermeij & Sandin, 2008). These stages are important links to recovery trajectories on coral reefs, because survivors will likely become competitive and reproductive adults in the population and contribute to live coral cover (Chong-Seng et al., 2014; Doropoulos et al., 2015; Hughes & Tanner, 2000; McClanahan et al., 2014).
The survival of early-life stages of corals depends on a series of biotic and abiotic factors that vary in space and time (Carleton & Sammarco, 1987; Dajka et al., 2019; Doropoulos et al., 2015; Sebens, 1982). The abundance and distribution of substrate competitors (e.g. sponges and macroalgae) and/or calcareous coralline algae which provide the chemical cues for coral larval settlement are all believed to affect recruitment and survivorship of juvenile corals (Dajka et al., 2019; Sato, 1985; Vermeij, 2006). Likewise, the habitat structural complexity has been proven to have a positive relationship with coral juveniles and recruits’ presence (Carleton & Sammarco, 1987; Dajka et al., 2019). Moreover, life history traits such as growth rate and morphology are key factors for corals determining early survivorship (Sato, 1985; Vermeij, 2006).
In the past, the study of coral recruits (i.e., new colonies recorded during consecutive periods [Caley et al. 1996]) and juveniles (i.e., each coral colony below or equal to 4 cm in diameter [Vermeij et al., 2011]) relied on the ability of divers to spot corals in the field, which can be challenging, expensive, and logistically complicated. Recent advances in digital photography, such as Structure from Motion technology (SfM), have allowed the creation of accurate 3-dimensional (3D) models of reef sections (Figueira et al., 2015) to study the dynamics of corals during their early stages (e.g. growth rates as in Ferrari et al., 2017). This technique has revolutionized the study of coral reef ecology, from polyps to landscapes (Gutierrez-Heredia et al., 2016; Martínez-Quintana et al., 2023; Urbina-Barreto et al., 2020).
This paper aims to evaluate the temporal and spatial dynamics of coral recruits and juveniles, and the surrounding benthic community in Playa Mero (PM), a section of Las Animas Cay within Morrocoy National Park, Venezuela. Historically estimated loss of living corals in this Marine Protected Area varies from 60 to 90% since 1996 (Laboy-Nieves et al., 2001; Villamizar, 2000). Former reef-building coral species in MNP and other Caribbean reefs such as Orbicella spp. were the main affected species by the massive mortality event that occurred in 1996 (Edmunds & Elahi, 2007; Laboy-Nieves et al., 2001; Villamizar, 2000), A survey conducted in 2017, showed a community dominated by macroalgae (Miyazawa et al., 2019). Using SfM technology to build 3D models of the habitat, we monitored the abundance and survivorship of juvenile corals and recruits on eight 2500 cm2 quadrats to see if changes in the benthic community structure and the structural complexity explained spatial and temporal changes of these juvenile and newly settled corals over 18 months. We also tested if the probability of survivorship was determined by growth rates of specific coral genera.
OBJECTIVES
Despite the rapid decline of coral reef health in Morrocoy, there is limited information about the recruitment and survivorship of juvenile and coral recruits, both being important variables for the dynamics of this degraded ecosystems.
- The objectives of the study were to: 1) understand the spatial and temporal variability of juvenile corals and benthic community in Playa Mero reef at a spatial scale of centimeters and a temporal scale of months, 2) determine which biotic and abiotic factors of the substrate affect the abundance of juvenile corals at the scale of time and space studied, 3) observe the recruitment dynamics in Playa Mero Reef at the temporal and spatial scale studied, 4) model the relationship between the survivorship of the juvenile and the mean growth rates.
MATERIALS AND METHODS
Study Area
Playa Mero (PM) (10.8 N; -68.2 E) is a fringing reef located inside the boundaries of Morrocoy National Park (MNP) which is the largest Marine Protected Area (MPA) on the Venezuelan western coast (Villamizar, 2000; Weil, 2003). The area is seasonally exposed to terrestrial runoff from the Aroa, Tocuyo, and Yaracuy rivers. Two large human settlements are located near the MPA; one in Chichiriviche and the other at Tucacas. Intense fishing, tourism, and coastal development have been ongoing for decades (Bone et al., 2005). The area has also had a long history of chemical pollution from industrial operations that have expanded over the last 60 years.
Experimental design
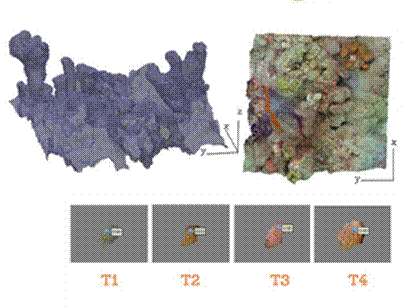
We determined spatial and temporal variability on the abundance, mortality, and survivorship of juvenile corals and benthic composition over eight haphazardly placed 50x50 cm quadrats, along the reef scape. The quadrats were delimitated by 4 metal bars, each one representing a vertex from the quadrat, and marked with individual underwater tags. All the quadrats were followed over 18 months starting in March 2017 (T1), and monitored in September 2017 (T2), February 2018 (T3), and August 2018 (T4). Thus, each quadrat represents a unique timeline for a 2500 cm2 area, and therefore, conclusions are only valid for that section of the reef.
Data collection in the field
For each quadrat, 2-to-3-minutes videos were filmed with a GoPro Hero 3TM following the lawnmower procedure outlined by Young et al. (2017) with slight modifications. To reduce lens distortion, Structure from Motion (SfM) based techniques require loop closure by starting and finishing the video always at the same point. Two videos for each quadrat were taken following the contour of the reef: one at 1 m and the other at 50 cm from the substrate to capture finer-scale details. A known reference scale was placed for the posterior scaling in each quadrat.
Data processing in the laboratory
Data processing in the laboratory consisted of 3 steps 1) Video processing 2) Construction of 3D models and orthomosaics 3) Data extraction from the 3D models and orthomosaics.
Video processing
Every video was converted into still frames by sampling at 3 Hz using the software FFmpeg (http://www.ffmpeg.org/), to obtain a 60-80% overlap between consecutive images (Young et al., 2017). Color balance was empirically adjusted and calibrated for every batch of images using XNConvert (freely available at https://www.xnview.com/en/xnconvert/).
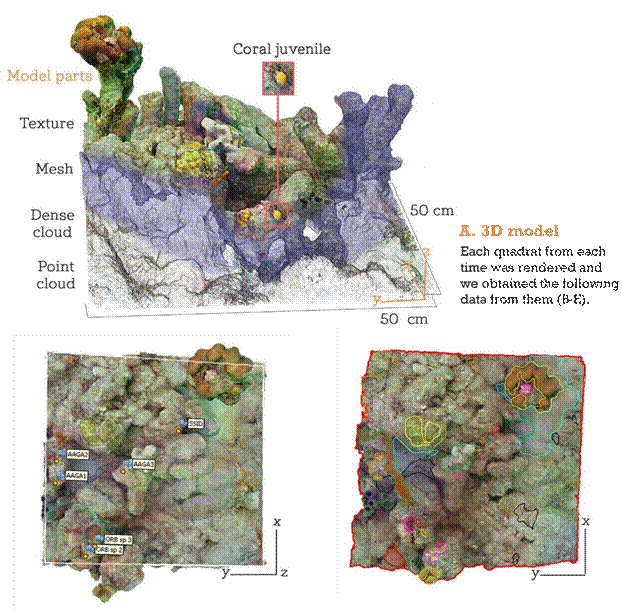
B. Abundance, survivorship, C. Benthic community recruitment Biotic and abiotic elements were censured in
 Each
individual was tagged followed the orthomosaics. The area of
each element through time in the 3D models. was selected in Photoquad
Software.
Each
individual was tagged followed the orthomosaics. The area of
each element through time in the 3D models. was selected in Photoquad
Software.
D. Rugosity
Ratio between surface area from mesh and orthomosaic area.
E. Growth rate
Surface area from each juvenile from each time was calculated.
Figure 1. Scheme used to obtain ecological data from the 3D models.
Construction of 3D models and orthomosaics
We produced the 3D models with Agisoft Photoscan Professional v 1.4.3 (Agisoft Software, 2016) from the colour-corrected images for each quadrat. Following standard workflow (Young et al., 2017), we first aligned the photos, then create the dense point cloud and from that we built the mesh, which is the geometric representation (Agisoft Software, 2016) of the quadrat surface. The final step consisted of adding texture to the model, that gives the realistic view of the surface adding the colors and textures. Detailed procedures explaining pipelines to generate 3D models in coral reef studies can be found in (Burns & Delparte, 2017; Ferrari et al., 2017; Figueira et al., 2015; Young et al., 2017).
During the post-processing phase, each model was rescaled using the reference placed during the video acquisition step. Then, the corners were identified and tagged to delineate the 50x50 cm2 polygon of the quadrat. Models were then rotated to align the corners with positive X and Y axis (Figueira et al., 2015). Finally, models were trimmed down by only selecting the area within the quadrat for posterior analyses (Fig. 1). The quadrat reconstruction models are available at sketchfab: https://sketchfab.com/gloria_mb/models.
For each model generated, we produced an orthomosaic, the orthogonal projection on a planar area of the model, selecting the “Orthomosaic Building” option in the Agisoft Software.
Data extraction from the 3D models and orthomosaics
Temporal and spatial changes in the abundance of juvenile corals
A visual census was conducted on each model to locate all present juvenile corals. Each individual bellow 4 cm in diameter was marked with the Agisoft Photoscan Professional marker tool and assigned a unique code for tracking over time. Coral juveniles were identified at the genus level (Vermeij et al., 2011).
Recruitment
To determine if Playa Mero provides suitable conditions for the settlement and survival of recruits, a visual survey was conducted to find settled coral individuals after time one. A recruit was defined in this work as a new individual that appeared between one time and another. Each recruit was marked with a unique identification code within the 3D model, and its development was tracked over time to determine whether it survived or not. Based on these data, the number of recruits that did not survive until the final time point was calculated by genus.
Benthic community structure
The substrate elements were surveyed for each quadrat at each time through the generated orthomosaics. Using the Photoquad program (Trygonis & Sini, 2012) the orthomosaics were scaled, and with the “Freehand ROI” tool each element of the substrate was delimitated by hand drawing a line around and then assigning the area inside to different categories (Fig. 1C). All substrate elements were surveyed following the functional group approaches for benthic community elements (Littler et al., 1983) macroalgae fleshy and calcareous, crustose coralline algae (CCA), turf, cyanobacteria. Dictyota spp. was identified because of its competitive importance with corals in the Caribbean (Box & Mumby, 2007). The other categories were sponges, anemones, octocorals, and scleractinian coral cover. The abiotic elements were grouped as sand and limestone debris, while unidentified elements, both biotic and abiotic, were categorized as “unknown elements.” A comprehensive survey of all substrate elements was conducted rather than using random points due to the spatial scale of the work (cm).
Surface rugosity
Another characteristic of the environment included in this analysis was surface rugosity, defined as the ratio between the mesh surface area (i.e., the three-dimensional area of the object or scene) and its orthogonal projection onto the surface plane (i.e., the two-dimensional area). This was represented by Equation 1, adapted from Friedman et al. (2012). The mesh surface area was calculated using the Agisoft PhotoscanPro program, while the orthogonal projection area was measured from the orthomosaic generated in Agisoft Photoscan and measured in the PhotoQuad software.
![]()
Equation 1. Surface rugosity equation taken from Friedman et al. (2012). Where A = mesh surface area and A’ = orthogonal projection area.
Survivorship of juveniles
To study the survival dynamics of juvenile corals, surviving corals (i.e., individuals present at T1 and at the end of the experiment, at T4) and non-surviving corals (i.e., juvenile corals present at T1 but not at T4) were counted. As each individual had a unique identification code, survivors and non-survivors were identified in the abundance matrix.
Measurement of growth rates
The measurement of growth rates was based on changes in surface area in each individual between one time and the next. The surface area measurement tool in Agisoft PhotoscanPro was used. For each time and quadrat, each juvenile coral was cropped, and its surface area was measured. The growth rate was calculated for each individual using Equation 2. Regarding the corals that died, the growth rate was calculated using the area of T1 and the area from the time before their death. The average growth rate was used to obtain more information about growth behavior.

Equation 2. Growth rate for the individual “X” between two adjacent times, where T = Time; AX(Ti+1) = Surface area of the individual “X” at time Ti+1; AX(Ti) = Surface area of the individual “X” at time Ti; and M = Number of months between Ti and Ti+1.
Statistical analysis
The data acquisition and statistical analysis stage is explained below. For all statistical analyses, a significance level of p < 5% or p < 0.05 and 999 permutations were used for ANOSIM analyses.
Temporal and spatial changes in the juvenile corals’ abundance
To study if the variability in juvenile coral abundance was determined by the factor of time and/or space (i.e., different locations of the quadrats on the reef), an Analysis of Similarities without replication (ANOSIM; Clarke & Gorley, 2006) was conducted. A Bray-Curtis similarity matrix was constructed for the analysis using Primer 6 software (Clarke & Gorley, 2006).
Relationships between substrate characteristics and coral juvenile abundance
To determine the substrate variables (both biotic and abiotic) that best predicted the spatiotemporal variability of juvenile coral abundance, a Distance Based Linear Model (DistLM, Anderson et al., 2008) was used. This routine allows modeling the relationships between a multivariate data cloud described in the similarity matrix with one or more variables (Anderson et al., 2008). In this study, we constructed a Bray-Curtis similarity matrix. The predicted variables were the substrate elements (biotic and abiotic) along with surface rugosity. The routine allows for the adjustment of individual or combined variables, providing information on how much each predictive variable explains the behavior of the response variables (i.e., marginal tests) or how much variation in the response variables can be explained together by all the predictive variables (i.e., sequential tests) (Anderson et al., 2008). This analysis was conducted using the PERMANOVA + software of PRIMER 6 (Anderson et al., 2008).
Relationship between survivorship of juvenile corals and growth rates
To further investigate the differences between individuals who survived and those who did not, a logistic logit model (Hilbe, 2015) was used to establish whether the survival probability of individuals depended on their genus or their growth rate. A fraction of the previously collected abundance data was chosen for analysis. The experimental units were the groups of quadrats at each time point, and the sampling units were the juvenile coral individuals of each genus with at least three individuals, thus excluding Colpophyllia spp. from the analysis. The factors studied were the genera of juvenile corals (with six levels) and growth rates (continuous values). Logistic models are suitable for studying survival because they use binary response values, such as the success or failure of a particular attribute. In this case, successful response was defined as the survival of an individual (variable taking a value of 1), and failure was the lack of survival (variable taking a value of zero). The survival predictors were the genera and the averages of the growth rates.
The effects package (Fox & Hong, 2009; Fox & Weisberg, 2019) in R V 3.6.0 was used (R Development Core Team, 2019). An important condition to run logit models is that the predictors are not correlated. Therefore, before this test, an ANOVA was run in R to determine if the variability in the average growth rates was significantly related to the genera of the individuals. It was found that the genera did not significantly explain the differences in the growth rates (p > 0.05), so the logit model was used.
RESULTS
Patterns of temporal and spatial changes in the abundance of juvenile corals
A total of 83 juvenile coral colonies belonging to seven genera were found in Playa Mero during the study period. ANOSIM test showed spatial (ρav = 0.537, p = 0.001) and temporal (ρav = 0.387, p = 0.001) statistical differences in the composition of juvenile corals in PM. Our results show that only a few genera managed to settle and/or reach sizes above 4 cm in PM. The genus Agaricia spp. dominated the juvenile coral community, with the total number of individuals per each period varying from approximately 30 to 40 (Fig. 2), approximately four times more individuals than the other genera.
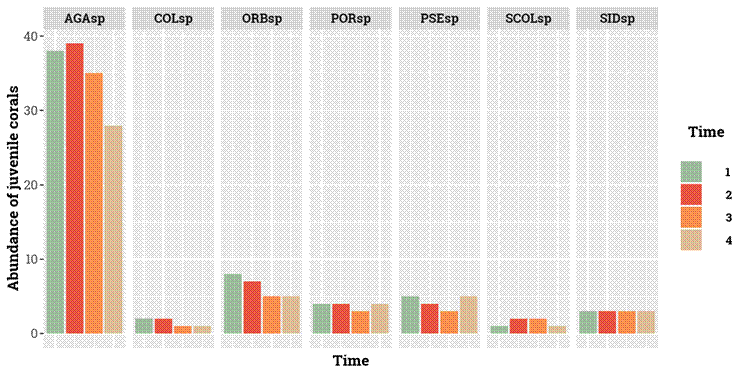
Figure 2. Total abundance of juvenile corals in Playa Mero Reef for 18 months and in eight 25 cm2 quadrats. AGAsp = Agaricia spp., COLsp= Colpophyllia spp., ORBsp= Orbicella spp., PORsp= Porites spp., PSEsp= Pseudodiploria spp., SCOLsp= Scolymia spp., SIDsp= Siderastrea spp.
Relationships between substrate variables and coral juvenile abundance
In the DistLM relating juvenile coral abundance to other features of the substrate, the marginal tests showed that macroalgae, sand, scleractinian coral cover and rugosity independently correlated with variation of the juvenile coral community through time and space (Table I). Fleshy macroalgae alone explained nearly 23% of the variability in the juveniles’ abundance, followed by calcareous macroalgae (near 16%). When all the variables are considered together in the sequential tests, the model explained up to 46% (Adj R2 = 0.46) of the spatial and temporal variability of juvenile corals at PM (Table II). The combination of macroalgae, sand, coral cover and surface rugosity explained most of the variation in this model, with up to 38%.
Table I. Marginal tests from de DistLM to predict juvenile coral abundance from substratum elements. SS(Trace)= Sum of squares; Pseudo-F= DistLM statistic, analog to Fisher’s F; P= Type Error I probability; Prop= Variance explained percentage by each variable. The variables denoted by bold characters, hold statistical significance (p< 0.05).
|
Variable |
SS(Trace) |
Pseudo-F |
P |
Prop (%) |
|
Actinaria |
1187.2 |
0.764 |
0.538 |
0.0248 |
|
Crustose coralline algae |
1084.7 |
0.696 |
0.565 |
0.0227 |
|
Cyanobacteria |
2763.1 |
1.84 |
0.132 |
0.0578 |
|
Dictyota spp. |
2357.3 |
1.55 |
0.169 |
0.0493 |
|
Octocorals |
326.7 |
0.206 |
0.935 |
0.0683 |
|
Calcareous macroalgae |
7836.5 |
5.88 |
0.001 |
16.4 |
|
Fleshy macroalgae |
10895 |
8.85 |
0.001 |
22.8 |
|
Sponges |
2689.7 |
1.79 |
0.143 |
0.0562 |
|
Rubble |
2058.8 |
1.35 |
0.266 |
0.0431 |
|
Sand |
5787.2 |
4.13 |
0.003 |
12.1 |
|
Scleractinian corals |
4851.8 |
3.39 |
0.014 |
10.1 |
|
Turf |
2479 |
1.64 |
0.174 |
0.0519 |
|
Surface rugosity |
5079.3 |
3.57 |
0.012 |
10.6 |
|
Unknown elements |
496.96 |
0.315 |
0.872 |
0.0104 |
Table II. Sequential tests from de DistLM to predict juvenile coral abundance from substratum elements. Adj R2= Adjusted R2; SS(Trace)= Sum of squares; Pseudo-F= DistLM statistic, analog to Fisher’s F; P= Type Error I probability; Prop= Variance explained percentage by each variable; Res.df= Degrees of freedom. The variables denoted by bold characters, hold statistical significance (p< 0.05).
|
Variable |
Adj R2 |
SS(trace) |
Pseudo-F |
P |
Prop (%) |
Cumul. |
Res.df |
|
Fleshy macroalgae |
0.136 |
7836.5 |
5.88 |
0.004 |
0.163 |
0.163 |
30 |
|
Calcareous macroalgae |
0.189 |
3726.9 |
2.98 |
0.039 |
0.078 |
0.242 |
29 |
|
Surface rugosity |
0.262 |
4389.3 |
3.86 |
0.012 |
0.092 |
0.334 |
28 |
|
Sand |
0.305 |
2923.2 |
2.73 |
0.067 |
0.061149 |
0.394 |
27 |
|
Scleractinian corals |
0.387 |
4072.8 |
4.26 |
0.011 |
0.0852 |
0.480 |
26 |
|
Dictyota spp. |
0.378 |
876.5 |
0.914 |
0.407 |
0.0183 |
0.498 |
25 |
|
Cyanobacteria |
0.387 |
1301.6 |
1.38 |
0.271 |
0.0272 |
0.526 |
24 |
|
Crustose coralline algae |
0.412 |
1851.5 |
2.04 |
0.139 |
0.0387 |
0.564 |
23 |
|
Actinaria |
0.437 |
1751.3 |
2.02 |
0.147 |
0.0366 |
0.600 |
22 |
|
Octocorals |
0.447 |
1184.3 |
1.39 |
0.232 |
0.0248 |
0.626 |
21 |
|
Rubble |
0.457 |
1170.6 |
1.40 |
0.266 |
0.0245 |
0.650 |
20 |
|
Turf |
0.448 |
551.32 |
0.648 |
0.554 |
0.0115 |
0.662 |
19 |
|
Sponges |
0.495 |
2159.7 |
2.78 |
0.085 |
0.0452 |
0.707 |
18 |
|
Unknown elements |
0.461 |
112.39 |
0.135 |
0.967 |
0.0235 |
0.705 |
17 |
Survivorship of juvenile corals and growth rates
Regarding the survivorship of juvenile corals, about 40% (n= 24) out of 61 individuals recorded in March 2017 died by August 2018. While linear growth rates did not statistically differ among genera (Table III), the logistic regression model showed this variable explained the overall chances of survivorship of any given juvenile colony (Nagelkerke R2= 0.67) (Table IV). The estimate of the variable mean growth rate is b= 5.225 which is positive, therefore an increase in the mean growth rate will increase the possibility of survivorship in the individual.
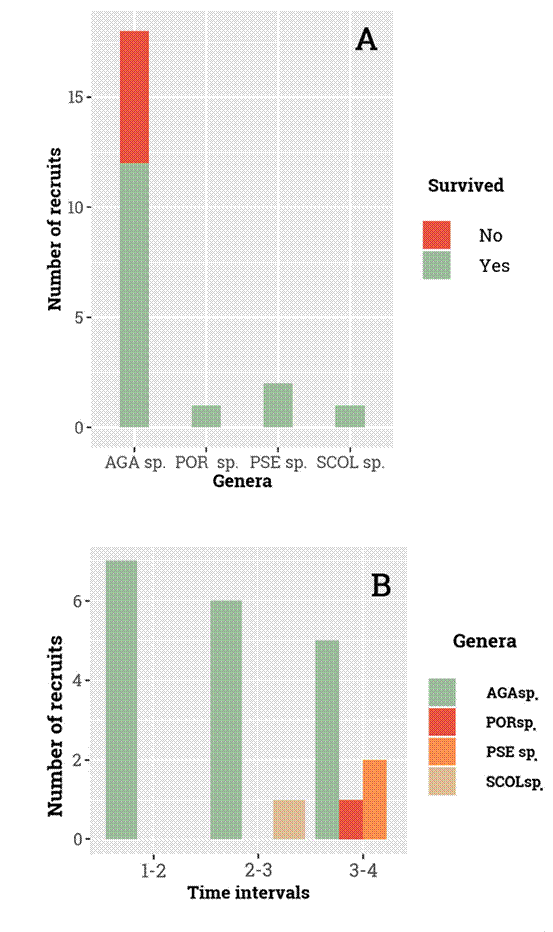
Figure 3. Recruiting patterns at Mero Reef during the study. A, total abundance of the recruits by genus and their survivorship until T4. B, changes in abundance over time per genus. AGAsp. = Agaricia sp., PORsp. = Porites sp., PSEsp. = Pseudodiploria sp., SCOLsp. = Scolymia sp.
Recruitment
We recorded 22 new colonies recruiting in our permanent quadrats, with the genus Agaricia spp. accounting for 82% of the new settlers for the 18-month period. From these recruits, 5 of them died during the study, and all of them belonged to the genus Agaricia. (Fig. 3A). New colonies of species in the genus Pseudodiploria, Scolymia and Porites were seldom recorded recruiting in PM (Fig. 3B). Our results indicate that only a few genera recruited during the study period with the genus Agaricia spp. being the genus with the highest number of new settlers but largest mortality during the study period.
Table III. ANOVA testing if the variability in growth rates can be explained by genus. Df= Degrees of freedom; Sum sq= Sum of squares; Mean sq= Mean square; F value= Fisher’s statistic; Pr(>F) = Probability of making a type I error.
|
|
Df |
Sum sq |
Mean Sq |
F value |
Pr(>F) |
|
Genera |
5 |
6.28 |
1.256 |
0.802 |
0.554 |
|
Residual |
53 |
83.04 |
1.567 |
|
|
Table IV. Logit model output. Estimate= the intercept (b0) and the beta coefficient associated to each variable; Std. Error= the standard error of the coefficient variables; z value= the z-statistic; Pr(<|z|) = P value corresponding to the z-statistic. The only significant variable for predicting survivorship in juvenile corals at Playa Mero were the mean growth rates.
|
(Intercept) |
16.53 |
2728.420 |
0.006 |
0.99528 |
|
Agaricia |
-15.973 |
2728.420 |
-0.006 |
0.99533 |
|
Orbicella |
-14.470 |
2728.421 |
-0.005 |
0.99577 |
|
Porites |
-14.798 |
2728.421 |
-0.005 |
0.99567 |
|
Pseudodiploria |
-17.571 |
2728.426 |
-0.006 |
0.99486 |
|
Mean growth rate |
5.225 |
1.636 |
3.193 |
0.00141 |
DISCUSSION
In this work, we utilized photogrammetry to study the change in coral communities at Playa Mero (PM) for 18 months. Our goal was to understand the ecological dynamics of juvenile and coral recruits from a disturbed reef in the Southern Caribbean. Our results showed that the variation in coral juvenile abundance was explained up to 46% by some benthic elements, such as fleshy algae and rugosity. Furthermore, there was recruitment of corals over 18 months, but these were dominated by a single genus (Agaricia spp.). Overall, this study demonstrated the strength of 3D reconstruction methodology in the detection and tracking of juvenile corals.
Patterns of temporal and spatial changes in the abundance of juvenile corals
Similar to other studies, this work reports the predominance of brooding species (e.g., Agaricia spp.), above species recognized as reef builders (e.g., Bak & Engel, 1979; Hughes & Tanner, 2000; Rogers et al., 1984; Vermeij et al., 2011). The most abundant genus in the juvenile community was Agaricia spp., but this genus had low adult coverage, according to a survey made the same year of this study (Miyazawa, 2019). Bastidas et al. (2006) visited another reef in MNP damaged by the same mortality event, Playa Caimán, where they also did not find some correspondence in the patterns of recruit abundance with the coverage of adult scleractinian corals in 2003. They discovered that over half of the the juvenile species were absent as adults. The dominance of Agaricia spp. in juvenile coral communities is not unusual in Caribbean coral reefs (Bak & Engel, 1979; Van Moorsel, 1985; Vermeij et al., 2011). This can be explained, among other reasons, by some characteristics of their life history. For example, Agaricia agaricites is a moderate sediment rejector (Bak & Engel, 1979) and tends to grow faster than other species, so they are expected to be better adapted to invade new substrates after disturbance (Hughes & Jackson, 1985). Despite the relative success in the juvenile life phase, it has been reported that Agaricia agaricites and other Agaricia spp. species such as Agaricia humilis are easily damaged and highly susceptible to mortality (Bak & Engel, 1979; Hughes & Jackson, 1985; Van Moorsel, 1985). This study shows that this species was the most recruited but also had the highest mortality recorded. This could explain its low cover as adult in the Playa Mero reef.
Relationships between substrate variables and coral juvenile abundance
The benthic elements commonly discussed in the literature as predictors for juvenile corals were established as factors predicting the patterns of juvenile abundance on the Playa Mero reef. Macroalgae, are significant reducers of juvenile coral abundance (Edmunds & Carpenter, 2001). They can make the substrate unsuitable for recruitment due to competition for space (Kuffner et al., 2006), directly damaging or killing juvenile corals by overgrowing them or through allelopathy (Edmunds & Carpenter, 2001; Sato, 1985; Vermeij et al., 2009). The presence of live coral cover, particularly the presence of certain genera surveyed in this study (e.g. Siderastrea spp. and Agaricia spp.), has been positively related to the abundance of juvenile corals. Vermeij (2005) demonstrated that larvae of Siderastrea siderea settle near parental colonies. This can partly explain why the presence of adult Siderastrea siderea corals is an important predictor of juvenile abundance patterns in Mero. Vermeij (2005) also found that the survival rate decreased near parental colonies and suggested that this could be because survival is dependent on density among recruits. This could explain the low abundance of Siderastrea spp. juveniles observed in the study.
Roughness, as an element of the topographic complexity of the substrate, was also important in determining juvenile abundance. Substrate irregularities and roughness have been positively correlated with the abundance patterns of benthic sessile organisms at various latitudes (Babcock & Mundy, 1996; Connell, 1961; Kuklinski et al., 2006), and specifically, with the abundance and survival of juvenile corals (Carleton & Sammarco, 1987; Gallagher & Doropoulos, 2017). Martínez-Quintana et al. (2023) found in the US Virgin Islands that roughness was an important predictor of recruitment of scleractinian corals. Greater roughness is associated with an increase in surface area (e.g., due to the presence of cracks and caves (Luckhurst & Luckhurst, 1978), and this can lead to a potential increase in microhabitat diversity (Sebens, 1991). Different microhabitats will vary the exposure of juvenile corals to predators (Gallagher & Doropoulos, 2017) or the flow of water currents over them that can lead to changes in the concentration, movement, or deposition of sediments around juveniles (Davis & Barmuta, 1989). But some studies have not found a relationship between the success of juveniles (i.e., survival) and different types of microhabitats (Edmunds et al., 2004; Roth & Knowlton, 2009). According to Edmunds et al. (2004), these negative results could be caused by spatial events at larger spatial scales that drive juvenile mortality. The mixed results found in the literature highlight the need to follow coral individuals over the long term to establish the cause-and-effect relationship between the structural complexity elements of the reef and the ecological dynamics of juvenile corals.
Survivorship of juvenile corals and growth rates
We found in this study that the success in the survival race in juvenile corals was defined by growth rates, regardless of their genus. However, Edmunds (2007) found differences in growth rates among juvenile coral taxa in the long-term experiment on the Caribbean Island of Saint John. Probably, in the study conducted in Playa Mero, there was a low number of samples with few replicates, as the survivors’ data was obtained as subsamples of the original data. This could mean a lack of statistical power for this test. On the other hand, Hughes & Tanner (2000) discovered that the probability of survival within the same coral species depended on the size class, with the smaller ones being more vulnerable to death. In this regard, Edmunds (2007) reported that the variability of growth rates in the same species and the same initial size class was highly variable. Therefore, the study of growth rates may be more accurate if factored by species or genus and size class, rather than just genus. Growing faster can determine whether a colony survives competition with macroalgae (Ferrari et al., 2012) or other coral colonies (Zilberberg & Edmunds, 2001). Hence, this characteristic possibly allows corals to reach faster the sizes at which they can overcome environmental and biological stress present in Mero.
Recruitment
During the study period, recruitment occurred, yet the recruits' community displayed limited diversity and differed significantly from the adult coral community in 1996 (Villamizar, 2000). Agaricia species dominated the recruits while Orbicella sp. or other typical Caribbean reef builders were entirely absent at the time and depth of the investigation. Agaricia spp. have notably high and consistent recruitment rates, whereas Orbicella spp. have been reported to exhibit low or absent recruitment (Hughes & Jackson, 1985; Hughes & Tanner, 2000; Miller et al., 2000; Woesik et al., 2014). This disparity might stem from their reproductive strategies; Agaricia species release planulae with multiple reproductive cycles yearly (Van Moorsel, 1983), while Orbicella spp. spawn gametes only once or rarely twice annually (Van Veghel, 1994), affecting their capacity to colonize disturbed areas primarily reliant on successful sexual reproduction events (Szmant, 1986).
The observed dynamics of coral juveniles and recruits at Playa Mero indicate a remote likelihood of the reef restoring its pre-1996 coral-dominated community. Understanding the drivers and mechanisms perpetuating these dynamics stands as the subsequent pivotal step in comprehending Mero’s recovery stagnation. These dynamics may stem from Morrocoy’s chronic disturbance regime, prevalent since the early 1970s (Weiss & Goddard, 1977). Disturbance factors outlined in literature affecting recruitment and juvenile survival such as sedimentation (Babcock & Smith, 2000; Fabricius, 2005, Tuttle & Donahue, 2022; Van Moorsel, 1985), hydrocarbon pollution (Hartmann et al. 2015), and presence of diseases (Cróquer et al., 2022) are present in MNP (Bastidas et al., 1999; Bone et al., 2005; Cróquer & Bone, 2003; García et al., 2011; Jaffé et al., 1998; Latchinian et al., 2017; Weiss & Goddard, 1977).
CONCLUSIONS AND LIMITATIONS
In conclusion, this study elucidated the dynamics of juvenile and coral recruits in Playa Mero, revealing key patterns: (1) juvenile abundance dominated by non-reef-building species, exemplified by Agaricia spp.; (2) a fluctuating juvenile community with an overall declining trend in space and time; (3) absence of reef-building coral recruits; (4) net recruitment rates lower than juvenile coral mortality; and (5) limited recruitment of species. The persistence of these dynamics is proposed to potentially lead to the collapse of the remaining coral community at Playa Mero. Literature evidence supports the notion that chronic disturbances spanning over 60 years in PNM may be linked to the dynamics of Mero’s recruits and juveniles, further studies should be followed to determine if and how these stresses are affecting coral early stages dynamics. However, while this paper seeks to explain temporal changes in recruitment using a deterministic approach, corals at this stage of their life cycle are likely driven by stochastic dynamics. Moreover, despite the limitations of 3D model analysis (i.e., paucity of light in areas partly shadowed where crevices are abundant and recruits often settle), we showed that photogrammetry is a useful tool to study the dynamics of coral during their early life stages as they digitally store the reef’s state, offering extended observation time and the option to revisit raw data.
ACKNOWLEDGMENTS
We would like to thank to all the members of Grupo de Investigación de Mecatrónica, especially to Manuel González and Novel Certad for helping in the recovery of this thesis data. Also, we are grateful with FUDENA NGO, especially with Samuel Narciso, for helping us in the field work and providing us stay. We are thankful to Jon Lefcheck because of his thoughtful comments about this manuscript and to Luis Miguel Montilla because of his comments and Latex instructions. We would also like to give thanks to Rita Peatchy and the 39th ALMC committee for supporting our travel to Dominican Republic to present this work to the Caribbean scientific community. The permit for taking the videos at MNP was given by Ministerio del Poder Popular para el Ecosocialismo y Aguas - Dirección General de Diversidad Biológica under the office no. 0033.
REFERENCES
Agisoft Software. (2016). Agisoft PhotoScan User Manual. Professional Edition, Version 1.2:37.
Anderson, M. J., Gorley, R. N., & Clarke, K. R. (2008). PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods. In: Plymouth, UK. 1–214.
Babcock, R., & Mundy, C. (1996). Coral recruitment: Consequences of settlement choice for early growth and survivorship in two scleractinians. Journal of Experimental Marine Biology and Ecology, 206(1–2), 179–201. https://doi.org/10.1016/S0022-0981(96)02622-6
Babcock, R., & Smith, L. (2000). Effects of sedimentation on coral settlement and survivorship. Marine Biology, I, 1–4.
Bak, R. P. M., & Engel, M. S. (1979). Distribution, abundance and survival of juvenile hermatypic corals (Scleractinia) and the importance of life history strategies in the parent coral community. Marine Biology, 54, 341–352. https://doi.org/10.1007/BF00395440
Bastidas, C., Bone, D., & García, E., M. (1999). Sedimentation rates and metal content of sediments in a Venezuelan coral reef. Marine Pollution Bulletin, 38(1), 16–24. https://doi. org/10.1016/S0025-326X(99)80007-1
Bastidas, C., Croquer, A., & Bone, D. (2006). Shift of dominant species after a mass mortality on a Caribbean reef. In: Proceedings of the 10th Int. Coral Reef Sym Coral Reef Symp. Okinawa, Japan. 989–993.
Birkeland, C., Rowley, D., & Randall, R. H. (1981). Coral recruitment patterns at Guam. In: Fourth International Coral Reef Symposium, Manila. Manila, 339–344.
Bone, D., Spiniello, P., Solana, P., Martín, A., García, E., López, J., La Barbera, A., Gómez, S., Pérez, D., Vera, B., Barreto, M. B., Zoppi, E., Miloslavich, P., Bitter, R., Klein, E., Villamizar, E., Losada, F., & Posada, J. (2005). Estudio integral del sistema Parque Nacional Morrocoy con vías de planes de uso y gestión para su conservación. Caracas.
Box, S. J., & Mumby, P. J. (2007). Effect of macroalgal competition on growth and survival of juvenile Caribbean corals. Marine Ecology Progress Series, 342, 139–149. https://doi.org/10.3354/meps342139
Burns, J. H. R., & Delparte, D. (2017). Comparison of commercial structure-from-motion photogrammety software used for underwater three-dimensional modeling of coral reef environments. The International Archives of the Photogrammetry, Remote Sensing and Spatial Information Sciences, 42, 127–131.
Caley, M. J., Carr, M. H., Hixon, M. A., Hughes, T. P., Jones, G. P., & Menge, B. A. 1996. Recruitment and the local dynamics of open marine populations. Annual Review of Ecology and Systematics, 27, 477–500. https://doi.org/10.1146/annurev.ecolsys.27.1.477
Carleton, J. H., & Sammarco, P. W. (1987). Effects of substratum irregularity on success of coral settlement: quantification by comparative geomorphological techniques. Bulletin of Marine Science, 40(1), 85–98.
Chong-Seng, K. M., Graham, N. A. J., & Pratchett, M. S. (2014). Bottlenecks to coral recovery in the Seychelles. Coral Reefs, 33, 449–461. https://doi.org/10.1007/s00338-014-1137-2
Clarke, K. R., & Gorley, R. N. (2006). PRIMER v6: Use manual/Tutorial. Plymouth: PRIMER-E Ltd.
Connell, J. H. (1961). Effects of competition, predation by Thais lapillus, and other factors on natural populations of the Barnacle Balanus balanoides. Ecological Monographs, 31(1), 61–104. https://doi.org/10.2307/1950746
Connell, J. H., Terrence, H., & Wallace, C. C. (1997). A 30-Year Study of Coral Abundance, Recruitment, and Disturbance At Several Scales in Space and Time. Ecological Monographs, 67, 461–488. https://doi.org/10.1890/0012-9615(1997)067[0461:AYSOCA]2.0.CO;2
Cróquer, A., & Bone, D. (2003). Las enfermedades en corales escleractínidos: ¿Un nuevo problema en el arrecife de Cayo Sombrero, Parque Nacional Morrocoy, Venezuela? Revista de Biologia Tropical, 51, 167–172.
Croquer, A., Zambrano, S., King, S., Reyes, A., Sellares Blasco, R. I., Valdez Trinidad, A., Villalpando, M., Rodriguez-Jerez, Y., Vargas, E., Cortes-Useche, C., Blanco, M., CalleTriviño, J., García-Camps, R., Hernández-Orquet, A., Torres, R., Irazabal, I., Díaz, L., Evangelista, Y., & Miyazawa, E. (2022). Stony Coral Tissue Loss Disease and Other Diseases Affect Adults and Recruits of Major Reef Builders at Different Spatial Scales in the Dominican Republic. Gulf and Caribbean Research, 33(1), GCFI1-GCFI13. https://doi.org/10.18785/gcr.3301.03
Dajka, J. C., Wilson, S. K., Robinson, J. P. W., Chong-Seng, K. M., Alasdair, H., & Graham, N. A. J. (2019). Uncovering drivers of juvenile coral density following mass bleaching. Coral Reefs, 38, 63–649. https://doi.org/10.1007/s00338-019-01785-w
Davis, J. A., & Barmuta, L. A. (1989). An ecologically useful classification of mean and near‐bed flows in streams and rivers. Freshwater Biology, 21(2), 271–282. https://doi. org/10.1111/j.1365-2427.1989.tb01365.x
Doropoulos, C., Ward, S., Roff, G., González-Rivero, M., & Mumby, P. J. (2015). Linking Demographic Processes of Juvenile Corals to Benthic Recovery Trajectories in Two Common Reef Habitats. PLoS ONE, 10(5): e0128535. https://doi.org/10.1371/journal. pone.0128535
Edmunds, P. J. (2007). Evidence for a decadal-scale decline in the growth rates of juvenile scleractinian corals. Marine Ecology Progress Series, 341, 1–13. https://doi.org/10.3354/ meps341001
Edmunds, P.J., Bruno, J. F., & Carlon, D. B. (2004). Effects of depth and microhabitat on growth and survivorship of juvenile corals in the Florida Keys. Marine Ecology Progress Series, 278, 115–124. https://doi.org/10.3354/meps278115
Edmunds, P. J., & Carpenter, R. C. 2001. Recovery of Diadema antillarum reduces macroalgal cover and increases abundance of juvenile corals on a Caribbean reef. Proceedings of the National Academy of Sciences, 98(9), 5067–5071. https://doi.org/10.1073/pnas.071524598
Edmunds, P. J., & Elahi, R. (2007). The demographics of a 15-year decline in cover of the Caribbean reef coral Montastraea annularis. Ecological Monographs, 77(1), 3–18. https:// doi.org/10.1890/05-1081
Edmunds, P. J., Steneck, R., Albright, R., Carpenter, R. C., Chui, A. P. Y., Fan, T. Y., Harii, S., Kurihara, H., Legendre, L., Mitarai, S., Muko, S., Nozawa, Y., Padilla-Gamino, J., Price, N. N., Sakai, K., Suzuki, G., van Oppen, M. J. H., Yarid, A., & Gates, R. D. (2015). Geographic variation in long-term trajectories of change in coral recruitment: A global-to-local perspective. Marine and Freshwater Research, 66(7), 609–622. https://doi.org/10.1071/
MF14139
Fabricius, K. E. (2005). Effects of terrestrial runoff on the ecology of corals and coral reefs: Review and synthesis. Marine Pollution Bulletin, 50(2), 125–146. https://doi.org/10.1016/j. marpolbul.2004.11.028
Ferrari, R., Figueira, W.F., Pratchett, M.S., Boube, T., Adam, A., Kobelkowsky-Vidrio, T., Doo, S. S., Atwood, T. B., & Byrne, M. (2017). 3D photogrammetry quantifies growth and external erosion of individual coral colonies and skeletons. Scientific Reports, 7, 16737.
https://doi.org/10.1038/s41598-017-16408-z
Ferrari, R., Gonzalez-Rivero, M., & Mumby, P. J. (2012). Size matters in competition between corals and macroalgae. Marine Ecology Progress Series, 467, 77–88. https://doi.org/10.3354/ meps09953
Figueira, W., Ferrari, R., Weatherby, E., Porter, A., Hawes, S., & Byrne, M. (2015). Accuracy and precision of habitat structural complexity metrics derived from underwater photogrammetry. Remote Sensing, 7(12), 16883–16900. https://doi.org/10.3390/rs71215859
Fox, J., & Hong, J. (2009). Effect displays in R for Multinomial and Proportional-Odds Logit Models: Extensions to the effects Package. Journal of Statistical Software, 32(1), 1–24. https://doi.org/10.18637/jss.v032.i01
Fox, J., & Weisberg, S. (2019). An R Companion to Applied Regression, 3rd Edition.
Friedman, A., Pizarro, O., Williams, S. B., & Johnson-Roberson, M. (2012). Multi-scale measures of rugosity, slope and aspect from benthic stereo image reconstructions. PLoS ONE 7(12), e50440. https://doi.org/10.1371/journal.pone.0050440.
Gallagher, C., & Doropoulos, C. (2017). Spatial refugia mediate juvenile coral survival during coral–predator interactions. Coral Reefs, 36, 51–61. https://doi.org/10.1007/s00338-0161518-9
García, E. M., Bastidas, C., Cruz-Motta, J. J., & Farina, O. (2011). Metals in waters and sediments of the Morrocoy National Park, Venezuela: increased contamination levels of cadmium over time. Water Air Soil Pollut, 204, 609–621. https://doi.org/10.1007/s11270-010-0450-9 Gutierrez-Heredia, L., Benzoni, F., Murphy, E., & Reynaud, E. G. (2016). End to end digitisation and analysis of three-dimensional coral models, from communities to corallites. PLoS ONE, 11(2), e0149641. https://doi.org/10.1371/journal.pone.0149641
Hartmann, A. C., Sandin, S. A., Chamberland, V. F., Marhaver, K. L., De Goeij, J. M., Vermeij, M. J. A. (2015). Crude oil contamination interrupts settlement of coral larvae after direct exposure ends. Marine Ecology Progress Series, 536, 163–173. https://doi.org/10.3354/ meps11437
Hilbe, J. M. (2015). Practical Guide to Logistic Regression. Boca de Ratón: Taylor & Francis Group, LLC. https://doi.org/10.18637/jss.v071.b03
Hilbe, J. M. (2015). Practical Guide to Logistic Regression (1st ed.). Chapman and Hall/CRC. https://doi.org/10.1201/b18678
Hughes, T. P. (1994). Catastrophes, phase shifts, and large-scale degradation of a Caribbean coral reef. Science, 265(5178), 1547–1551. https://doi.org/10.1126/science.265.5178.1547
Hughes, T. P., Graham, N. A. J., Jackson, J. B. C., Mumby, P. J., & Steneck, R. S. (2010). Rising to the challenge of sustaining coral reef resilience. Trends in Ecology & Evolution, 25(11), 633–642. https://doi.org/10.1016/j.tree.2010.07.011
Hughes, T. P., & Jackson, J. B. C. (1985). Population dynamics and life histories of foliaceous corals. Ecological Monographs, 55(2), 141–166. https://doi.org/10.2307/1942555
Hughes, T. P., & Tanner, J. E. (2000). Recruitment failure , life histories , and long-term decline of Caribbean corals. Ecology, 81(8), 2250–2263. https://doi.org/10.2307/177112
Jaffé, R., Leal, I., Alvarado, J., Gardinali, P. R., Sericano, J. L. (1998). Baseline study on the levels of organic pollulants and heavy metlas in bivalves from the Morrocoy National Park, Venezuela. Marine Pollution Bulletin, 36(11), 925–929. https://doi.org/10.1016/s0025326x(98)00090-3
Kuffner, I. B., Walters, L. J., Becerro, M. A., Paul, V. J., Ritson-Williams, R., & Beach, K. S. (2006). Inhibition of coral recruitment by macroalgae and cyanobacteria. Marine Ecology Progress Series, 323, 107–117. https://doi.org/10.3354/meps323107
Kuklinski, P., Gulliksen, B., Lønne, O. J., & Weslawski, J. M. (2006). Substratum as a structuring influence on assemblages of Arctic bryozoans. Polar Biology, 29, 652–661. https://doi. org/10.1007/s00300-005-0102-5
Laboy-Nieves, E. N., Klein, E., Conde, J. E., Losada, F., Cruz, J. J., & Bone, D. (2001). Mass mortality of tropical marine communities in Morrocoy. Bulletin of Marine Science, 68(2), 163–179.
Latchinian, A., Dopazo, C., Porras, J. A., Reid, J., & Piñango, A. (2017). Elaboración de un Plan de Gestión Ambiental para el Parque Nacional Morrocoy, Venezuela. Gestión y Ambiente, 20(1), 22–37. https://doi.org/10.15446/ga.v20n1.59318
Littler, M., Littler, D., & Taylor, P. (1983). Evolution strategies in a tropical barrier reef system: Functional-form groups of marine macroalgae. Journal of Phycology, 19(2), 229–237. https://doi.org/10.1111/j.0022-3646.1983.00229.x
Luckhurst, E., & Luckhurst, K. (1978). Analysis of the influence of substrate variables on coral reef fish communities. Marine Biology, 49(9), 317–323.
Martínez‐Quintana, Á., Lasker, H. R., & Wilson, A. M. (2023). Three‐dimensional species distribution modelling reveals the realized spatial niche for coral recruitment on contemporary Caribbean reefs. Ecology Letters, 26(9), 1497–1509. https://doi.org/10.1111/ele.14281
McClanahan, T. R., Graham, N. A. J., & Darling, E. S. (2014). Coral reefs in a crystal ball: Predicting the future from the vulnerability of corals and reef fishes to multiple stressors. Current Opinion in Environmental Sustainability, 7, 59–64. https://doi.org/10.1016/j. cosust.2013.11.028
Miller, M. W., Weil, E., & Szmant, A. M. (2000). Coral recruitment and juvenile mortality as structuring factor for reef benthic communities in Biscayne National Park, USA. Coral Reefs, 19, 115–123.
Miyazawa E. (2019). Variabilidad espacial de las comunidades bentónicas arrecifales en Venezuela: la relevancia de las escalas. Universidad Simón Bolívar.
Van Moorsel, G. (1983). Reproductive strategies in two closely related stony corals (Agaricia, Scleractinia). Marine Ecology Progress Series, 13, 273–283. https://doi.org/10.3354/ meps013273
Van Moorsel, G. (1985). Disturbance and growth of juvenile corals {Agaricia humilis and Agaricia agaricites, Scleractinia) in natural habitats on the reef of Curagao. Marine Ecology Progress Series, 24, 99–112. https://doi.org/10.3354/meps024099
Mumby, P. J., & Steneck, R. S. (2011). The resilience of coral reefs and its implications for reef management. In: Dubinsky, Z., Stambler, N. (Eds.) Coral Reefs: An Ecosystem in Transition (pp 509–521). Springer, Dordrecht. https://doi.org/10.1007/978-94-007-0114-4_29
R Development Core Team. (2019). A language and environment for statistical computing. R Foundation for Statistical Computing.
Ritson-Williams, R., Arnold, S., Fogarty, N., Steneck, R. S., Vermeij, M., & Paul, V. J. (2009). New perspectives on ecological mechanisms affecting coral recruitment on reefs. Smithsonian Contributions to the Marine Sciences, 437–457. https://doi.org/10.5479/ si.01960768.38.437
Rogers, C. S., Fitz, H. C., Gilnack, M., Beets, J., & Hardin, J. (1984). Scleractinian coral recruitment patterns at Salt River submarine canyon, St. Croix, U.S. Virgin Islands. Coral Reefs, 3, 69–76. https://doi.org/10.1007/BF00263756
Roth, M. S., & Knowlton, N. (2009). Distribution, abundance, and microhabitat characterization of small juvenile corals at Palmyra Atoll. Marine Ecology Progress Series, 376, 133–142. https://doi.org/10.3354/meps07787
Sato, M. (1985). Mortality and growth of juvenile corals Pocillopora damicornis (Linnaeus). Coral Reefs, 4, 27–33. https://doi.org/10.1007/BF00302201
Sebens, K. P. (1982). Competition for space: Growth rate, reproductive output, and escape in size. The American Naturalist, 120(2), 189–197. https://doi.org/10.1086/283982
Sebens, K. P. (1991). Habitat structure and community dynamics in marine benthic systems. In: Bell, S. S., McCoy, E. D., & Mushinsky, H. R. (Eds) Habitat Structure. Population and Community Biology Series (pp 211–234). Springer, Dordrecht. https://doi.org/10.1007/97894-011-3076-9_11
Souter, D., Planes, S., Wicquart, J., Logan, M., Obura, D., & Staub, F. (2021). Status of coral reefs of the world: 2020.
Szmant, A. M. (1986). Reproductive ecology of Caribbean reef corals. Coral Reefs, 5, 43–53. https://doi.org/10.1007/BF00302170
Trygonis, V., & Sini, M. (2012). PhotoQuad: A dedicated seabed image processing software, and a comparative error analysis of four photoquadrat methods. Journal of Experimental Marine Biology and Ecology, 424–425, 99–108. https://doi.org/10.1016/j.jembe.2012.04.018.
Tuttle, L. J., & Donahue, M. J. (2022). Effects of sediment exposure on corals: a systematic review of experimental studies. Environmental Evidence, 11(1), 1–33. https://doi.org/10.1186/ s13750-022-00256-0
Urbina-Barreto, I., Chiroleu, F., Pinel, R., Fréchon, L., Mahamadaly, V., Elise, S., Kulbicki, M., Quod, J.-P., Dutrieux, E., Garnier, R., Bruggemann, J. H., Penin, L., & Adjeroud, M. (2021). Quantifying the shelter capacity of coral reefs using photogrammetric 3D modeling: From colonies to reefscapes. Ecological Indicators, 121, 107151. https://doi.org/10.1016/j. ecolind.2020.107151
Van Veghel, M. L. J. (1994). Reproductive characteristics of the polymorphic Caribbean reef building coral Montastrea annularis. I. Gametogenesis and spawning behavior. Marine Ecology Progress Series, 109(2/3), 209–219.
Vermeij, M. J. A. (2005). Substrate composition and adult distribution determine recruitment patterns in a Caribbean brooding coral. Marine Ecology Progress Series, 295, 123–133. https://doi.org/10.3354/meps295123
Vermeij, M. J. A. (2006). Early life-history dynamics of Caribbean coral species on artificial substratum: The importance of competition, growth and variation in life-history strategy. Coral Reefs, 25, 59–71. https://doi.org/10.1007/s00338-005-0056-7
Vermeij, M. J. A., Bakker, J., van der Hal, N., & Bak, R. P. M. (2011). Juvenile coral abundance has decreased by more than 50% in only three decades on a small Caribbean Island. Diversity, 3(3), 296–307. https://doi.org/10.3390/d3030296
Vermeij, M. J. A., & Sandin, S. A. (2008). Density-dependent settlement and mortality structure the earliest life phases of a coral population. Ecology, 89(7), 1994–2004. https://doi.
org/10.1890/07-1296.1
Vermeij, M., Smith, J., Smith, C., Vega Thurber, R., & Sandin, S. A. (2009). Survival and settlement success of coral planulae : independent and synergistic e V ects of macroalgae and microbes. Oceanologia, 159, 325–336. https://doi.org/10.1007/s00442-008-1223-7
Villamizar, E. (2000). Estructura de una comunidad arrecifal en Falcón, Venezuela, antes y después de una mortalidad masiva. Revista de Biologia Tropical, 47(S1), 19–30.
Weil, E. (2003). Coral and coral reefs of Venezuela. Latin American Coral Reefs, 303–330. https://doi.org/10.1016/B978-044451388-5/50014-X
Weiss, M. P., & Goddard, D. A. (1977). Man’s impact on coastal reefs. An example from Venezuela. In: Frost, S. H. Weiss, M. P. & Saunders, J. B. (Eds.) Reefs and Related Carbonates-Ecology and Sedimentology. Studies in Geology No. 4 (pp 11–124). American Association of Petroleum Geologists.
Woesik, R. Van, Iv, W. J. S., & Aronson, R. B. (2014). Lost opportunities: Coral recruitment does not translate to reef recovery in the Florida Keys. Marine Pollution Bulletin, 88(1–2), 110–117. https://doi.org/10.1016/j.marpolbul.2014.09.017
Young, G. C., Dey, S., Rogers, A. D., & Exton, D. (2017). Cost and time-effective method for multi-scale measures of rugosity, fractal dimension, and vector dispersion from coral reef 3D models. PLOS ONE, 12(4), e0175341. https://doi.org/10.1371/journal.pone.0175341
Zilberberg, C., & Edmunds, P. J. (2001). Competition among small colonies of Agaricia: the importance of size asymmetry in determining competitive outcome. Marine Ecology Progress Series, 221, 125–133. https://doi.org/10.3354/meps221125
Citation: Mariño-Briceño, G., Cappelletto, J., Ascanio, A., Agudo-Adriani, E., & Cróquer, A. (2024). Describing the dynamics of recruits and juvenile scleractinian corals using 3d models: a case study from Cayo Mero reef, Morrocoy National Park, Venezuela. Novitates Caribaea, (23), 51–73. https://doi.
org/10.33800/nc.vi23.347