![]() Número 23,
enero, 2024: 22–50
Número 23,
enero, 2024: 22–50
ISSN versión impresa: 2071–9841 ISSN versión en línea: 2079–0139 https://doi.org/10.33800/nc.vi23.346
THE RECENT HISTORY OF AN INSULAR BAT POPULATION
REVEALS AN ENVIRONMENTAL DISEQUILIBRIUM AND CONSERVATION CONCERNS
La historia reciente de una población insular de murciélagos revela un desequilibrio medioambiental y preocupaciones por su conservación
Corentin Bochaton1*, Rémi Picard2, David Cochard3a, Valentin Conche3b, Kevin Lidour4, and Arnaud Lenoble3c
1 Institut des Sciences de l’Évolution Montpellier ISEM, Université de Montpellier, IRD, CNRS, EPHE- Place Eugène Bataillon, CC 065 34095 Montpellier cedex 5, France, https://orcid.org/0000-0003-4954-0019. 2 FREDON Martinique, 218 chemin Tolobé, 97224, Ducos, Martinique, https://orcid.org/0009-0002-1762-8358, r.picard@fredon972.org. 3PACEA-UMR CNRS 5199 – Université de Bordeaux – Ministère de la Culture et de la Communication, Avenue Geoffroy St. Hilaire, CS 50 023, 33615 Pessac cedex, France, 3a https://orcid. org/0000-0002-4548-3769, david.cochard@u-bordeaux.fr; 3b https://orcid.org/0009-0008-9792-5881, valentin. conche@hotmail.fr; 3c https://orcid.org/0000-0001-9023-9741, arnaud.lenoble@cnrs.fr. 4Instituto Internacional de Investigaciones Prehistóricas de Cantabria (IIIPC). Universidad de Cantabria, Avenida de los Castros, 52, 39005 Santander, Spain, https://orcid.org/0000-0002-0252-9376, lidour01@gmail.com. *Corresponding author: corentin.bochaton@cnrs.fr.
[Recieved: November 06, 2023. Accepted: December 31, 2023]
ABSTRACT
With the global pandemic of Covid-19, the putative threats related to the increasing contact between wild animals, including bats, and human populations have been highlighted. Bats are indeed known to carry several zoonoses, but at the same time, many species are currently facing the risk of extinction. In this context, being able to monitor the evolution of bat populations in the long term and predict future potential contact with humans has important implications for conservation and public health. In this study, we attempt to demonstrate the usefulness of a small-scale paleobiological approach to track the evolution of an insular population of Antillean fruit-eating bats (Brachyphylla cavernarum), known to carry zoonoses, by documenting the temporal evolution of a cave roosting site and its approximately 250 000 individuals bat colony. To do so, we conducted a stratigraphic analysis of the sedimentary infilling of the cave, as well as a taphonomic and paleobiological analysis of the bone contents of the sediment. Additionally, we performed a neotaphonomic study of an assemblage of scats produced by cats that had consumed bats on-site. Our results reveal the effects of human-induced environmental disturbances, as well as conservation policies, on the bat colony. They also demonstrate that the roosting site is currently filling at a very fast pace, which may lead to the displacement of the bat colony and increased contact between bats and human populations in the near future. Our research outcomes advocate for a better consideration of retrospective paleobiological data to address conservation questions related to bat populations.
Keywords: Cave, French Antilles, Martinique, Island, paleoecology, taphonomy.
RESUMEN
Con la pandemia global de Covid-19, se han resaltado las amenazas putativas relacionadas con el aumento del contacto entre animales salvajes, incluidos los murciélagos, y las poblaciones humanas. Es cierto que los murciélagos son conocidos por portar varias zoonosis, pero al mismo tiempo, muchas especies enfrentan actualmente el riesgo de extinción. En este contexto, poder monitorear la evolución de las poblaciones de murciélagos a largo plazo y prever el futuro contacto potencial con los humanos tiene importantes implicaciones para la conservación y la salud pública. En este estudio, intentamos demostrar la utilidad de un enfoque paleobiológico a pequeña escala para seguir la evolución de una población insular de murciélagos frugívoros antillanos (Brachyphylla cavernarum), conocidos por portar zoonosis, documentando la evolución temporal de un sitio de descanso en una cueva y su colonia de aproximadamente 250 000 individuos. Para hacerlo, realizamos un análisis estratigráfico del relleno sedimentario de la cueva, así como un análisis tafonómico y paleobiológico del contenido óseo del sedimento. Además, llevamos a cabo un estudio neotafonómico de un conjunto de heces producidas por gatos que habían consumido murciélagos en el lugar. Nuestros resultados revelan los efectos de las perturbaciones ambientales inducidas por humanos, así como las políticas de conservación, en la colonia de murciélagos. También demuestran que el sitio de descanso se está llenando actualmente a un ritmo muy rápido, lo que podría llevar al desplazamiento de la colonia de murciélagos y a un aumento del contacto entre murciélagos y poblaciones humanas en el futuro cercano. Los resultados de nuestra investigación abogan por una mejor consideración de los datos paleobiológicos retrospectivos para abordar preguntas de conservación relacionadas con las poblaciones de murciélagos.
Palabras clave: cueva, Antillas Francesas, Martinica, isla, paleoecología, tafonomía.
INTRODUCTION
The interest in paleobiological data to address modern biodiversity issues has been increasingly pointed out in the context of the current environmental crisis (Barnosky et al., 2017; Boivin & Crowther, 2021; Dietl & Flessa, 2011). These approaches are especially relevant and impactful in islands whose Holocene ecosystems were often quickly anthropized and damaged before being studied by scientists (Hughes et al., 2023; Nogué et al., 2017) such as the Caribbean islands in which several studies already make use of the past record to address recent extinction and current conservation questions (Bochaton et al., 2021; Kemp & Hadly, 2015; Soto-Centeno & Steadman, 2015). Paleobiological approaches are most often the only way to describe the initial stages of natural environments and faunas before the impact of human colonization. In addition, as the relationship between health and environmental challenges is increasingly recognized such as with the “One Health” concept (Zinsstag et al., 2011), paleontological and historical data could also be of use to address past and even future sanitary issues. Regarding vertebrates, retrospective paleobiological data can, for instance, enable the documentation of the long-term evolution of wild animal populations which are possible carriers of zoonoses diseases. Such a study could have strong implications as the long-term increasing human pressure on natural environments progressively leads to an increase in the contact between human and animal populations, and thus of the risks of inter-specific transmission of diseases (Morand, 2020; Morand & Lajaunie, 2021). Recently, bats have been at the center of these preoccupations because, in addition to having many species currently facing extinction risks (Mickleburgh et al., 2002), they are known carriers of several diseases including coronaviruses diseases (Banerjee et al., 2019; Li et al., 2005) but also some that are known to be directly transmissible to human, such as histoplasmosis (Diaz, 2018). However, obtaining good-enough subfossil data to allow for a precise description of the evolution of a given faunal community, in the long run, is extremely challenging. Indeed, such approaches are mostly based on accumulations of subfossil bones. However, the composition of such assemblage is subject to many biases that mostly reflect the parameters of the accumulation processes of the bones (e. g. behaviors of a predator). For instance, even in caves that are currently occupied by bats, these animals might have been absent in the past and the fossil bone accumulation preserved in the substrate be made by a predator accumulator agent (Pedersen, Kwiecinski, et al., 2018). One of the ways to limit the biases impacting such study is to focus the investigation on very small chronological scales and geographic ranges in which the different parameters could be less susceptible to strong variations and in an in-depth study of a high-resolution paleobiological record is possible. This does not however mean that paleobiological approaches are unable to provide information relevant to tackle questions at broader scales as the evolution of the biological community in a single site can also reflect external large-scale phenomena.
In this study, we attempt to demonstrate the usefulness of a small-scale paleobiological approach to address broader biodiversity conservation and sanitary questions related to the Martinique Island population of Antillean fruit-eating bat (Brachyphylla cavernarum Gray, 1834). To achieve this goal, we try to reconstruct the recent history of a bat roosting site, the Chancel Cave located on the Chancel Islet, to document the evolution of the site and its bat colony through time. To do so, we use sedimentological and stratigraphic analysis of the sedimentary infilling of the cave as well as a taphonomic and paleobiological analysis of the bone contents of the sediment layers including an assemblage of animal scats collected in the stratigraphy of the site. Using the obtained paleobiological data we discuss the putative effects of environmental disturbances and conservation policies on the bat colony and address the question of its future evolution concerning potential sanitary issues related to the zoonotic pathogens the population is known to carry.
Regional setting
The Chancel Islet is a small volcanic island of 0.7 km² located 300 m from the Eastern Coast of Martinique Island in the Southern Lesser Antilles (Fig. 1). The Chancel Cave (60° 52.940’ W 14° 41.666’ N) is located in a cliff on the Northern coast of the Islet (Fig. 1), around 15 m above the sea level. The cave itself is around 30 m long, 20 m wide, and between 2 to 3.5 m high for a surface of around 444 m². Its opening is pretty narrow, less than 1.5 m high and 15 m wide. The cave is formed of volcanic puddingstone, a rock in which caves usually do not form. The formation process of the site remained thus unclear especially because the nature and content of the sedimentary infilling were never documented. The site is renowned to be hosting the biggest colony of Antillean fruit-eating bats (Brachyphylla cavernarum Gray, 1834) of Martinique. The first scientific testimony regarding the Chancel Cave and its bat colony dates back to 1979 when around 500 individuals of B. cavernarum were observed in the site (Magnaval, 1984) although the owner of the Chancel Islet indicates that the cave was already densely populated by bats at least several decades before that. Subsequent testimonies indicate the occurrence of several hundreds of bats in the cave in 1994, and a hundred in 1997 (Breuil, 1997).
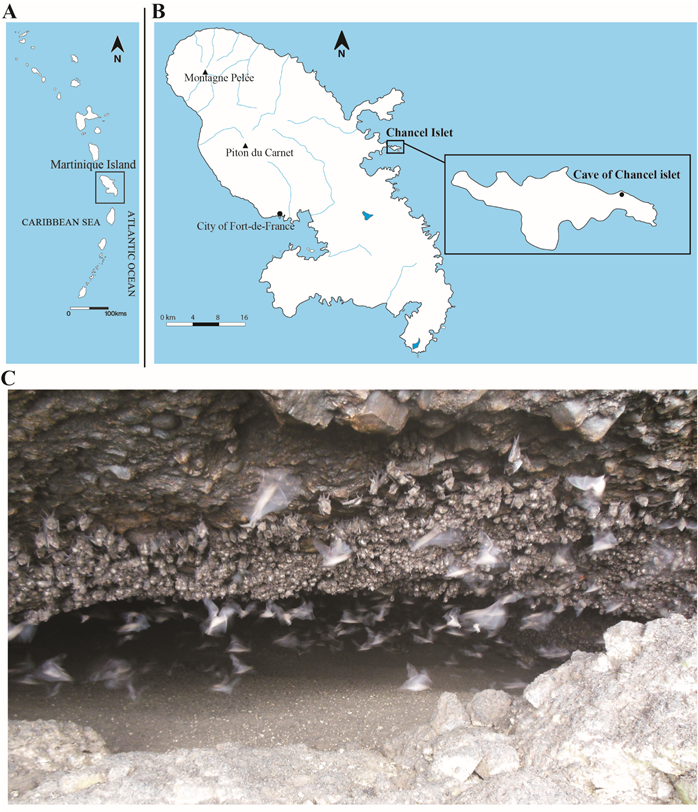
Figure 1. A) Map of the Lesser Antilles with the location of Martinique Island. B) Map of Martinique Island and the Chancel Islet with the location of the Chancel Cave. C) A picture of the entrance of Chancel Cave during the day showing B. cavernarum individuals being present very close to the entrance due to the lack of space on the cave’s roof inside.
For this last observation, B. cavernarum was the most abundant species but the occurrence of four additional species was signaled: Artibeus jamaicensis, Natalus stramineus, Pteronotus davyi, and Noctilio leporinus. In 1999, the cave was occupied by 5000 B. cavernarum and at least 1500 P. davyi (Issartel, 2000). In 2004, 5000 B. cavernarum, and 200 P. davyi were observed (Issartel & Leblanc, 2004). In 2010, the cave was occupied by at least 24 000 individuals of B. cavernarum, and no other species was observed (Rufray com. pers.). In 2012, some individuals of P. davyi have been observed in the cave which was otherwise full of individuals of B. cavernarum (Questel com. pers.). Since 2013, all the observers have indicated that the cave was completely occupied by bats but the indicated number of individuals suffers some variation depending on the methodology used to count them. Indeed, the colony has been successively estimated to 250 000 (Lenoble & Queffelec, 2016), 353 250 (Issartel & Jemin, 2017), and 300 000 individuals (Issartel & Jemin, 2017). Our observations in the course of our paleobiological excavations in 2017 indicate the occurrence of between 100 000 and 250 000 individuals of B. cavernarum in the cave (Picard, 2017). The size of the colony has thus critically increased since 2004 and now attained its maximum size as there is no place left on the ceiling of the cave during daylight when the bats are inside the site.
Regarding the occurrence of putative predators, M. Breuil reports the presence of hundreds of cat scats on the floor of the cave in 1994 as well as of at least six mummified cats (Breuil, 1997). A living cat has been observed on the site in 2012 (Questel com. pers.) and some rare cats have been observed on the Chancel Islet between 2006 and 2015 but never more than one or two individuals (Ourly, 2006; C. Rodriguez, com. pers.). Due to the systematic eliminations of these cats to preserve the iguana (Iguana delicatissima) population of the islet, the species was absent from the island at the time of our investigation in 2017 (M. Bally pers. com.). It is likely that the situation of the 90’s with several cats being present in the vicinity of the cave never happened again. The 1994 reports also indicate the occurrence of a rifle cartridge in the cave which indicates the possibility of human predation on the bat colony although this could also be related to the elimination of the cats (Breuil, 1997).
OBJECTIVES
- Study the long-term evolution of the bat population of Chancel Island.
MATERIALS AND METHODS
Excavation and recovery of bones and scats samples
Two excavation test pits have been performed on the site: one of 1 m² on the bench near the entrance of the cave (test pit 1), and one of 2 m² in the deepest part of the cave (test pit 2) (Fig. 2a). At the exception of a unique modern plastic artifact both test pit were free of manufactured remains. For logistical reasons, half of the sediment of only the first test pits has been fully sampled and divided into 12 spits 5 cm high each to allow for the search of small bone remains. To do so, 200 kg of sediment were water-sieved with 2 mm mesh, and the remaining bone elements were extracted and sorted to be studied. Samples of scat observed in the stratigraphy were also collected in both test pits to extract their bone content for additional studies.
After having been identified as cat scats based on their size and morphology, these scats were prepared individually by being soaked in water to enable their bone content to be manually extracted. The content of each scat has been isolated and studied. In total, 16 204 bone remains have been collected from the sediment of test pit 1, and 4365 from the scats. The sediment contained an important quantity of insect remains that were not collected. The stratigraphy of both test pits has been recorded, described, and drawn on-site. In both test pits, we reached a depth of respectively 0.6 m and 2 m before having to interrupt the excavation on a thick layer of scree that was impossible to pass through. A carbon-14 date (Lyon-15659(GrM)) has been performed on a sample of several bat bones collected at the base of the stratigraphy of test pit 1 (unit d) at the radiocarbon laboratory of Lyon. The 14C date obtained was calibrated using the software Oxcal v4.3.2 and the Intcal20 curve (Reimer et al., 2020).
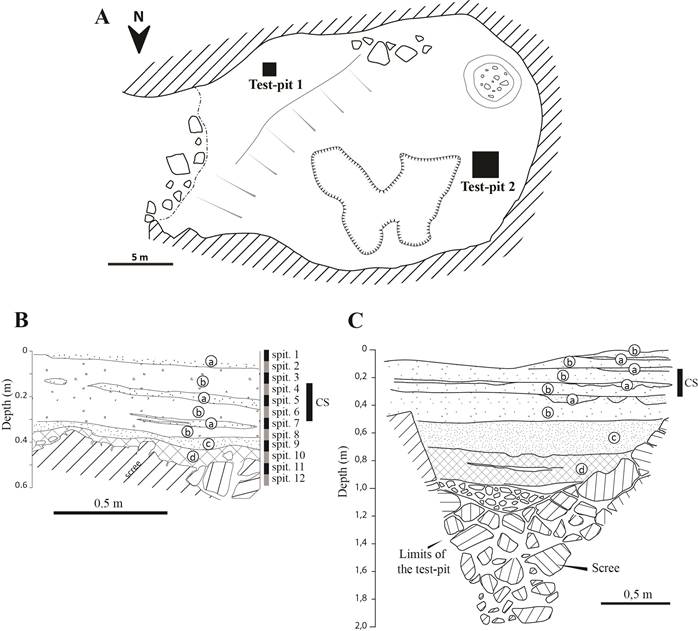
Figure 2. A) Map of the cave with the location of the two test pits. B) Stratigraphy of the first test pit. C) Stratigraphy of the second test pit. CS: Layers in which the cat scats were collected; a, b, and c are the different sedimentary facies described in the part of the result section dedicated to the stratigraphy of the cave.
Study of the bone assemblages
To follow the long-term evolution of the bat colony of the Chancel Cave in terms of taxonomic composition, number of individuals, size structure, predation pressures it might have been confronted with, as well as its use of the cave, we choose to perform detailed taxonomic, taphonomic, and paleobiological studies of the full sample of bones collected in the sediment and the scats. The general observation of the bones has been performed using a binocular microscope. The taxonomic identifications of the bones were obtained through a direct comparison of the recovered bones to modern individuals from the osteological comparative collection of the PACEA laboratory (Bordeaux, France). The morphology of B. cavernarum teeth and long bone has been used to define the maturity stage. We considered a specimen as immature as long as at least one of its teeth was not fully erupted or its roots not fully developed, and if at least one of the epiphysis of its long bones (humerus, radius, femur, and tibia) was still unfused to the diaphysis. These data were combined with the Minimal Number of Individuals (MNI) of each spit and scat, to determine the proportion of juvenile and mature individuals in each context. Generally in bats, the complete formation of the definitive teeth is synchronous with the starting of the flight of young individuals (Brunet-Rossinni & Wilkinson, 2009). There is however no study regarding the relationship between bone maturity and the first flight in B. cavernarum. In some species (e. g. Myotis lucifugus) the first flight occurs once the radius diaphysis is fully fused with both its extremities (Adams, 2000) but no data were collected on the other long bones although this should probably be also true for the bones that need to be rigid enough to support the physical constraints related to a flying activity (i.e. the humerus). Considering the proportion of bones of many different sizes we recorded as immature in comparison to the adult ones, it is possible that most subfossil specimens were not mature enough to fly. To make the interpretation of the data easier we thus choose to arbitrarily consider our juvenile age category as specimens who never flight even if the largest juvenile specimens might have been able to fly at least to some extent (Kunz & Anthony, 1982). The sex of the adult specimens has been identified when possible based on the morphology of the pelvic bone (Pelletier et al., 2017).
To investigate the anatomical distribution of the bone remains, a Proportional Representation (PR) (Dodson & Wexlar, 1979) of each considered anatomical part has been performed to compare the different spits of the test pit 1 and the samples collected in the scats. To do so, the count of each anatomical part has been divided by the number of occurrences of that given anatomical part in a complete skeleton. The obtained number was then divided by the Minimal Number of Individuals in the considered sample (e. g. a spit of the test pit). The Minimal Number of Individuals (MNI) has been defined using the frequency of the most abundant anatomical element in the considered sample (Lyman, 2008). Regarding the scats, to keep methodological homogeneity and to keep the comparison relevant with the material collected in the test pit, we defined the MNI using the full bone sample collected in all the scats and did not add up the MNI of each scat. The MNI of B. cavernarum in the stratigraphy of the test pit 1 has been estimated based on the number of radius bones recovered. In the scats, this number has been estimated based on the number of superior canines recovered. Both these anatomical elements were the best represented in their respective bone assemblages. Fragmentation of the remains has been registered on every bone element recovered from the scats and only on the humerus and radius of the bones collected in the sediment. For the bones collected in the sediment, we limited the documentation of the fragmentation to the basic condition of the bones (complete, sub-complete, or fragment) but for the scats, a completion rate (CR) corresponding to the percentage of the complete bone represented by each fragment has been estimated. This was made to allow for future comparisons with neotaphonomic studies. The maximal length of the complete juvenile and adult radius and humerus bones has been measured. The mixture analysis of the distribution of the bone sizes in the assemblage has been performed using the package “mixtools”(Young et al., 2016) on the open-source software R (https://cran.r-project.org/). The possible occurrence of digestion and tooth marks on the bones have been recorded using a binocular microscope on every bone attributed to a given anatomical part in the scat sample, and only on the humerus and radius bones collected in the sediment. To do so we followed the methodology and classification proposed by Stoetzel et al. (2021).
RESULTS
Stratigraphy and dating of the infilling
The infilling of the cave is similar in both test pits. Below the surface layer of fresh guano that has been removed before the excavation was a layer of fresh guano thick between 0.5 m (Test pit 1) and 1 m (Test pit 2) overlying a scree layer of unknown thickness. The guano layer displays four sedimentary facies (indicated in Fig. 2B and 2C): a) A medium fine grey sand rich in insects exoskeleton fragments of Tenebrionidae and Blattidae; b) An agglomerate of grey-brown multi-centimetric aggregates rich in hair, with a “Puerh cake” facies. The sediments on this facies are rich in chiropteran remains, represented by connected bones sometimes preserving the patagium of individuals with some rare fragments of elytra; c) A pseudo-sand formed of millimeter-sized fragments of light grey cylindrical droppings. The sediments on this facies are poor in chiropteran bones; d) A mass of sub-centimetric brown aggregates with felting linked to the abundance of hairs and presenting shimmering mineralizations, probably of gypsum. The organization of these facies is identical in the two test pits, with an alternation of the facies “a” and “b” in the upper part of the deposits, while the superposition of facies “c” and “d” characterizes the lower part of the guano layer (Fig. 2B and 2C). The preservation of bat specimens in the facies “b” is remarkable with the presence of partly complete bat mummies. However, a gradient of preservation is noted, with a progressive loss of the integrity of the desiccated skin of the specimens with depth. The astonishing preservation of organic elements in the guano layer is also made visible by the occurrence of preserved tree leaves, observed at a depth of 20 cm in test pit 1, and by the occurrence of connected cockroach exoskeletons observed below 50 cm of depth in the same test pit. Both test pits also yielded a stratigraphic horizon characterized by an abundance of cat scats at the base of the upper third of the guano layer (see CS in Fig. 2C and 2B). The same scree layer was encountered under the accumulation of guano in both test pits. It presents all the characteristics of a rockfall eboulis: open structure, unsorted blocks, random arrangement of elements, and fragmented block with a jigsaw fit (i.e. fractured blocks with no or limited displacement of the different fragments).
The most obvious particularity of the guano accumulation of the Chancel Cave is the very slow bacterial decomposition of organic matter. This is probably linked to the combined absence of humidity (dry cave) and the presence of salts in this coastal environment, playing an aseptic role (Magnaval, 1984). It is also likely that the very high ammonia content of the sediment, caused by the very high impregnation of the deposit with bat urine, also limits the development of microbiota. In these conditions, the decomposition has to be mostly done by the insect soil fauna present on the site (Tenebrionidae and cockroaches). Such activity can be linked to the formation of the facies “a” and “c” which are formed of droppings and fragments of these insects. Conversely, sediments on facies “b” are characterized by the absence of this decomposition process. There is thus a succession of phases when the guano is decomposed by soil fauna and phases when it is not, maybe in response to fluctuations in the abundance of insects in the cave. The cause of these fluctuations is however unknown. It could be a climatic signal (seasonal), a sign of natural events (sudden influx of salt into the cave during storms/hurricanes), or a variation in the number of bats roosting in the cave. In any case, the guano might not decompose during some periods in the cave which lead to a very high sedimentation rate caused by the quantity of guano created by the population of hundred thousand bats occupying the site. This is confirmed by the dating elements collected in the lower lens (facies d) of the guano layer, namely a plastic gun was made by the Gualandi company and circulating since the ’60s or ’70s, and a 14C date of 150 ± 30 BP (1667-1950 cal. AD) (Lyon-15659) obtained on bat bones in the same layer. These elements indicate that this lens might correspond to a longer period than the other layers that all formed in about 50 years. In addition, shimmering mineralizations (gypsum) in the facies “d” lens could indicate an incipient reaction horizon between the accumulation of guano and the rockfall eboulis (Hill & Forti, 1997). The acidification of this horizon and the resulting alteration of the bones it contains are all the more pronounced as the cave is formed in a volcanic breccia and, as such, no calcareous element buffers the action of acid solutions.
Analysis of the bone remains collected in the stratigraphy of the test pit 1
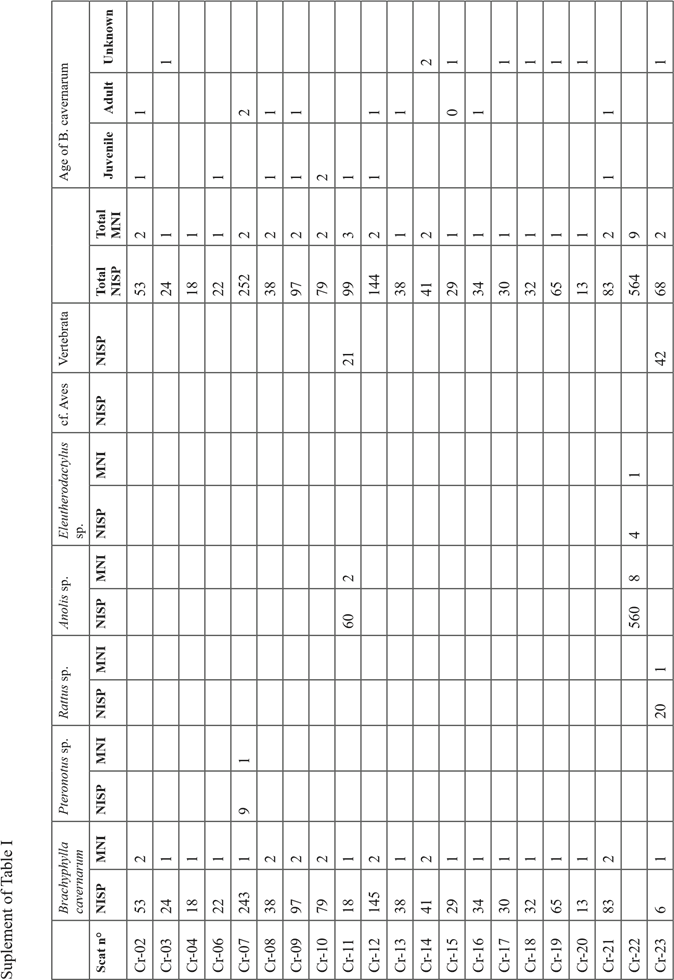
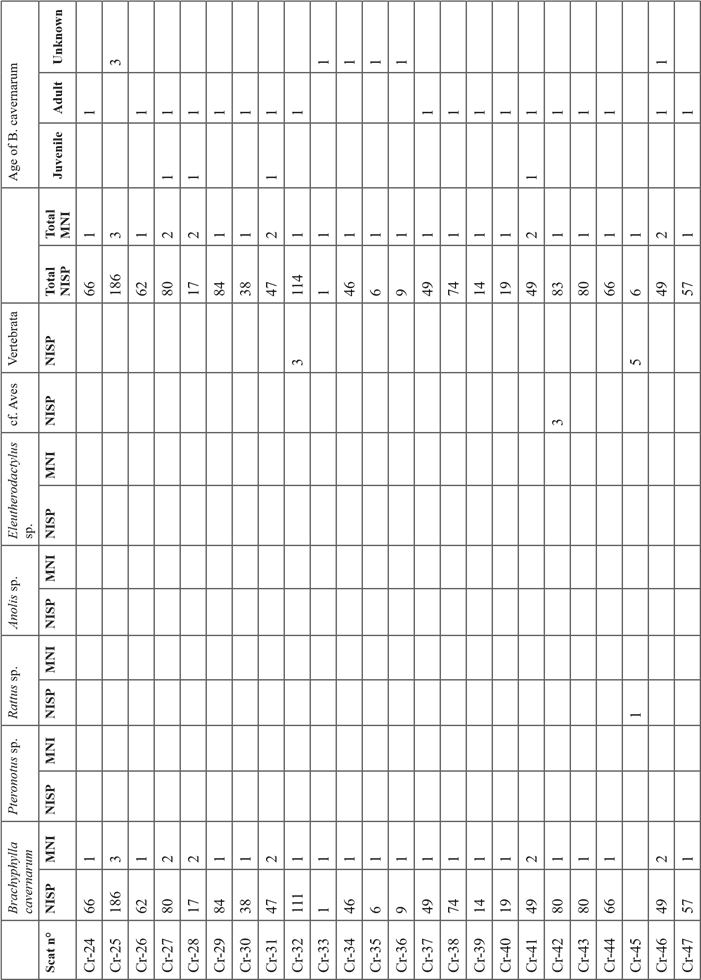
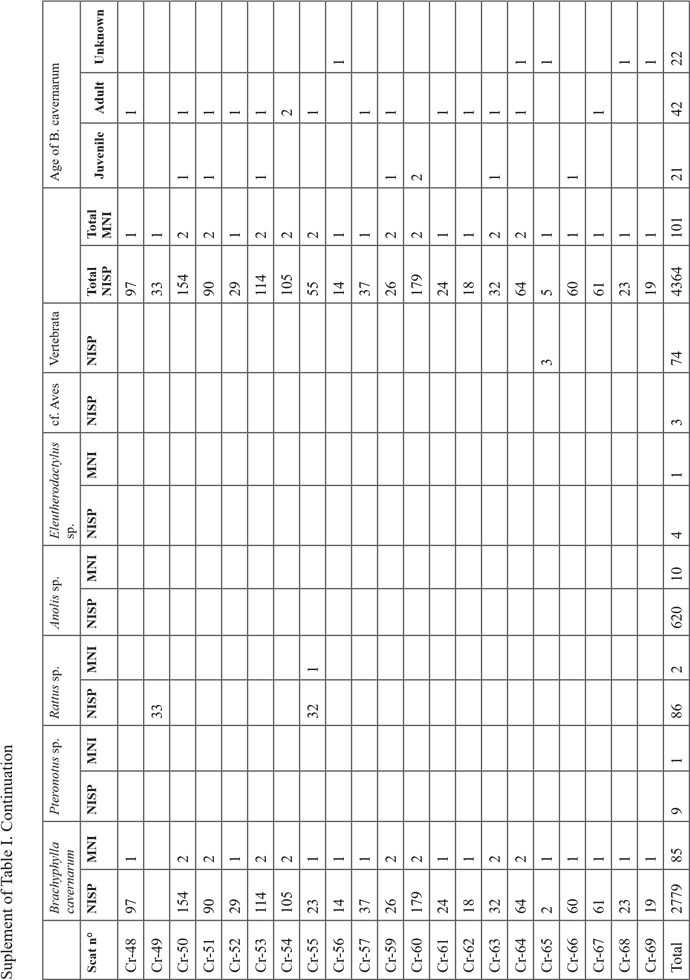
The 16 204 bone remains collected in the sediment of test pit 1 (this does not include the bones collected in the cat scats) were mostly located in the spits 1 to 7 with a better representation in facies “b” lenses (mean of 64 bones / dm3) than in the sedimentary facies “a” ones (mean of 42 bones / dm3) (Table I). Below the spit 7 the concentration of bone falls abruptly and become extremely low below the spit 8 in the facies “c”, and “d”.
In the sample, 99.9% of the collected bones were referred to as Brachyphylla cavernarum. The remaining vertebrate taxa are Pteronotus sp. represented by 5 bones in the upper facies “a” (spits 1 and 2), Mus musculus represented by 7 bones from the spits 2 and 7, and Rattus sp. represented by 7 bones (a mummified forelimb) in the spit 2. The minimal number of individuals of B. cavernarum in the whole assemblage is 380. In the first spit, the adult individuals of B. cavernarum are better represented than the juveniles with two adults for one juvenile (Table I). This tendency is reversed in every other spit where there is 1.9 juvenile for one adult in the second spit then between 2.7 to 6.6 juveniles for one adult in the subsequent spits. Determination of the sex of the B. cavernarum individuals was possible on 86 pelvic bones and indicated a ratio of 2.3 males for 1 female in the full assemblage but there was not enough sex data to enable a comparison between the different spits.
The length of the 104 measured fully fused and complete radius bones was between 57.5 and 64.7 mm and that of the 33 measured humerus bones was between 34.8 and 41.7 mm. The length of the unfused complete bones is between 16.7 and 56.8 mm for the radius and between 14.4 and 35 mm for the humerus. The size distribution of the adult radius is most likely bimodal as indicated by the results of a Hartigans’ dip test for unimodality (P-val. > 0.05) and of a mixture analysis (Fig. 3A). The sizes of the immature radius are more evenly distributed but larger individuals closer to maturity are better represented (Fig. 3B). There is no difference in the size of the radius collected in the different spits. The bimodality of the size distribution of adult radius is difficult to explain, B. cavernarum is not known to present a strong sexual dimorphism if any (Catzeflis et al., 2018), and the size difference could potentially correspond to the presence of lineages of different size in the caves.
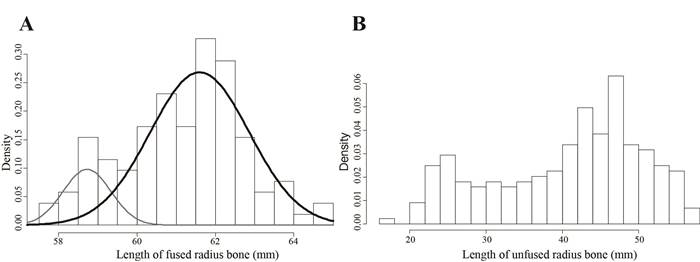
Figure 3. Size of adult (A) and immature (B) radius bones of B. cavernarum measured in the bone material collected in the test pit 1. The figure A shows the two normal populations resulting from the mixture analysis.
Regarding the anatomical distribution of the B. cavernarum remains, in every spit, we observed a tendency for a better representation of the anterior limbs toward the posterior ones and a strong under-representation of the smallest and most fragile anatomical parts (vertebrae, ribs, and clavicles) (Fig. 4A). This last tendency is the most pronounced in the deepest and thus oldest spits (8 and 9). The spit 1 was the most singular with its stronger representation of humerus, metacarpus, and anterior phalanx combined with a more pronounced scarcity of scapula bones and posterior elements. The mean PR of spit 1 (29%) was also lower than that of the others spits (43–35%). This is probably related to the abundance of the mummified bats’ wings collected in this layer which increased the MNI of this layer. Otherwise, the overall anatomical distribution of the bone elements is mostly similar in every spit.
The fragmentation of B. cavernarum humerus and radius is limited with an average of 66.3% of humerus and 77.6% of radius being complete or sub-complete in the assemblage. The fragmentation of the humerus is far stronger in spit 1 (16% of complete or sub-complete bones) and becomes weaker in spit 2 (51% of complete or sub-complete bones) and subsequent spits (71% of complete or sub-complete bones). Among the broken humerus, the distal part represents 125 of the 132 fragments and a clear breakage pattern has been observed in the material collected in spit 1 and 2 (Fig. 4A). Similar observations could be made on the radius which are more fragmented in the spits 1 and 8 (50% of complete or sub-complete bones) than in the other spits (between 70 and 94% of complete or sub-complete bones). Similarly, with the humerus, the distal part of the radius is also better represented than the proximal part with respectively 47 and 27 fragments. Putative digestion marks have been observed on 3.8% of the humerus and radius fragments of the sample (50 occurrences). Most of these traces are from spit 1 (N=36) and spit 2 (N=10). The rare tooth marks (N=9) were observed in spit 1 (N=6).
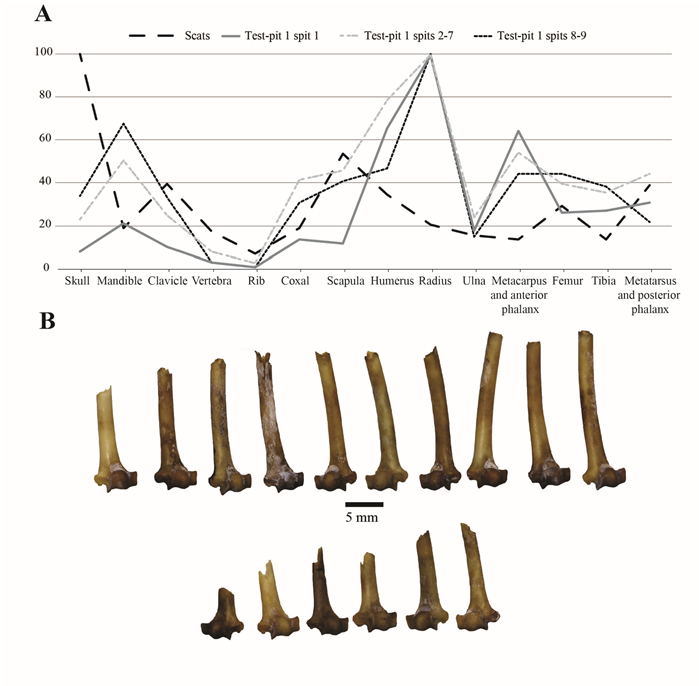
Figure 4. A) Anatomical distribution of the remains of B. cavernarum in the scats and the different spits of test pit 1. B) Broken humerus collected in spits 1 and 2 of test pit 1.
Analysis of the scats
The 67 analyzed scats contained between 1 and 564 bone remains (mean=65) for a total of 4365 bone remains (Sup. Tab. I). The assemblage is nearly only composed of remains of Brachyphylla cavernarum (NR=3569; MNI=29) a bat represented in nearly every scat (65/67) with some rare additional taxa. The only other bat represented in the assemblage is Pteronotus sp. (NR=9; MNI=1) which was identified in a single scat. Rodents are also represented with four scats containing remains of Rattus sp. (NR=86; NMI=2). Non-mammal vertebrate taxa are represented in two scats, one containing many remains of Anolis lizards (NR=560; MNI=9), and one containing bones of an Eleutherodactylus sp. frog (NR=4; MNI=1). Regarding the maturity of B. cavernarum individuals identified in the scats, one out of three (33%) was an immature individual (Sup. Tab. I).
Out of B. cavernarum, no taxon had well-represented enough remains in the scats to enable useful observations of the anatomical distribution of their bones in the assemblage. Regarding B. cavernarum, the skull was the best-represented anatomical part thanks to the high proportion of teeth in the assemblage (Fig. 4A). Overall, all the anatomical parts were represented and no anatomical parts were excluded from the assemblage. However, the smallest and bulkiest bones that are less likely to be fragmented and destroyed during the mastication and digestion processes are the best represented (teeth, proximal scapula, and posterior extremity). The slightly better representation of the axial and posterior parts of the skeleton might indicate that the wings of the prey were not consistently ingested by the predator but this could also only reflect a preservation difference. Regarding the humerus, there is no tendency for a better representation of the distal or proximal parts and thus no clear relation between the anatomical distribution of the remains observed in the scats and the complete bat wings collected in the sediment of the spit 1.
The average completion rate (CR) of the bones in the scats is 36% with the largest bones (e. g. skull: 6%, mandible: 24.5%, long bones: 13%) being much more fragmented than smaller ones (e. g. posterior phalanx 3: 91.5%; teeth: 90%). Only 11.4% of the bones of the overall sample were complete, 90% of these being teeth, metatarsus, and posterior phalanx. The overall breakage is very strong as attested by the fact that 1847 bone remains out of the 4365 composing the full assemblage were small fragments that could not be attributed to a given anatomical part. The strong fragmentation of the bones did not enable any measurement of the long bones or sexing of the specimens.
In the whole scats sample, 81% of the bones were presenting digestion marks ranging mostly from high (63%) to medium (27%) and low (9%) intensity (Fig. 5). These traces are distributed evenly on all anatomical parts at the exception of the terminal posterior phalanxes whom 36% lack any digestion marks thanks to the protection of their keratin sheath that was in many cases preserved in the scats.
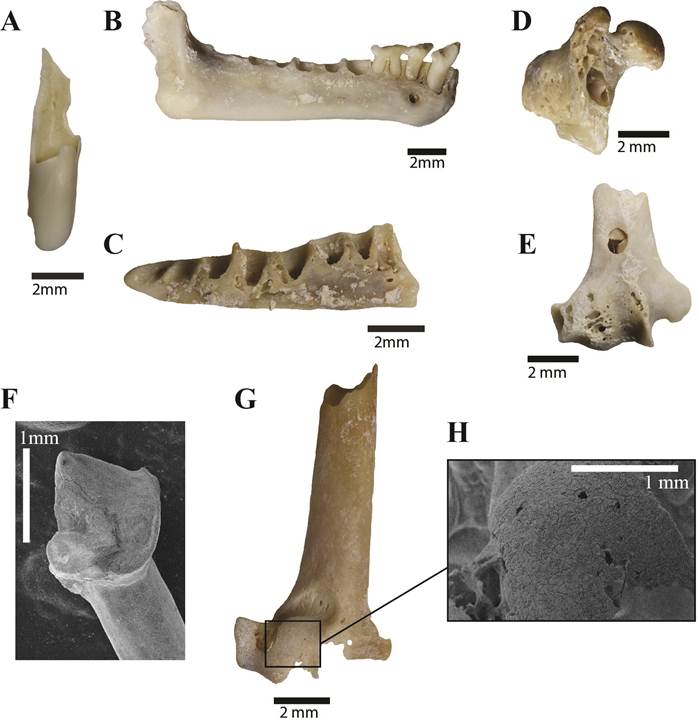
Figure 5. Digested Brachyphylla bones and teeth from the cat scat samples. A) Heavily digested long bone diaphysis; B) heavily digested right mandible; C) heavily digested left mandible; D) heavily digested proximal humerus; E) heavily digested distal humerus; F) heavily digested teeth with the enamel partly detached from the crown; G) heavily digested humerus; H) focus on the distal epiphysis of the picture G showing the surface state of the bone.
DISCUSSION
Accumulation process of the sedimentary infilling and the history of the cave
The sedimentological and faunal bone data collected in the stratigraphy indicate that the complete sedimentary infilling of the cave is composed of bat guano which is associated with a high frequentation of the cave by the Antillean fruit-eating bat (B. cavernarum), a species that is over-dominant in the bone assemblage. Juvenile specimens account for the majority of B. cavernarum individuals in every layer of the Chancel Cave infilling except for the first spit in which they only account for around 30% of the specimens. This unambiguously indicates that the cave has always and still functions as a maternity roost. Gravid B. cavernarum females usually leave their colony roosting site to establish maternity roosts which means that only some sites are used by females to give birth and take care of their newborn. In some cases, this segregation of the bats’ individuals can take place in a single site. However, the use of the Chancel Cave as a maternity roost was never evidenced during the observations made directly on the bat colony (Issartel & Jemin, 2017). An explanation could be a seasonality pattern in the exploitation of the cave by the colony. Indeed, the birthing period of B. cavernarum is from endApril to end-June (Pedersen, Kwiecinski, et al., 2018; Pedersen, Larsen, et al., 2018; Swanepoel & Genoways, 1983) while the cave has been visited during winter or at the beginning of the spring. Regarding the sex ratio of the adult B. cavernarum individuals studied in our subfossil bone assemblage, it is biased toward males with more than two males for one female. Such a ratio is not congruent with a maternity roost in which adult males should be absent (Bond & Seaman, 1958; Genoways et al., 2007, 2012) but is coherent with a site not working as such (Pelletier et al., 2017). This discrepancy could be explained by the position of the test pit from which the bones were collected, near the entrance of the cave. Indeed, in some sites, both male and female individuals are present in maternity sites but with spatial segregation. In such sites, females occupy the deepest parts of the roost (Genoways et al., 2007). It is thus possible that the Chancel site is widely used throughout the year with spatial segregation of the male and female inside the cave at least during the birthing period.
Apart from the cat droppings collected in the sediments and the bone they contain, the paleobiological and taphonomic data indicate that the bone remains collected in most of the stratigraphy correspond to an attritional mortality of bats roosting in the cave. Most of these bones are complete and lack predation marks. Another indication is the fact that most represented specimens found in the sediment are juvenile individuals. The taphonomic observations made on the scat samples indicate that the bone contents of the layers of test pit 1 do not correspond to the content of dislocated scats. The size distribution of the immature radius does however not correspond to a typical attritional mortality profile. Indeed, it has been documented that the mortality of juvenile bats is much higher in newborns (Foster et al., 1978; Hermanson & Wilkins, 1986) and decreased over time until maturity. Our size profile shows the exact opposite with an increased occurrence of larger individuals in the bone samples. Apart from the cat scats, there is however no evidence of predation in the material below the spit 2. Thus, this strong representation of large immature individuals could reflect a preservation bias with the bone of the youngest individuals being less well preserved and may have been destroyed by scavengers because of their poor calcification state. The cave is currently occupied by an important population of American cockroaches (Periplaneta americana) that are known to consume and destroy bones (Parkinson, 2012), and we observed feeding from bats carcasses in the cave.
Another explanation for the absence of newborns in the bone assemblage could also be that these individuals were located in other areas of the cave maybe in the deepest parts from which we did not collect bone samples. The taphonomy of the bone assemblage is in any case different in the uppermost part of the infilling (spit 1–2) in which several clear pieces of evidence of predation are present in addition to the attritional mortality profile visible in the other spits. These two most-upper spits were also the only one to contain remains of a second species of bats (Pteronotus sp.) but their bones remains scars in respect to the proportion of this species observed in the cavity between 1999 and 2010. This could have several explanations including different use of the cave by the different bat species.
The spits 4–6 contained many cat scats whose content was nearly only composed of B. cavernarum remains. Our results indicate that the cats who produced these scats were consuming all the body parts of both adult and juvenile bats. This evidence of predation from the scats is associated with some predation marks in the material collected in the sediment but this phenomenon is difficult to characterize on the bone assemblage collected in the sediment. At the time of the formation of these layers, we can confidently say that the cave was exploited by cats. This population of cats was probably heavily reliant on the bat colony given the content of the scats and also the fact that Breuil described the cats present in the cave in 1994 as starving which indicates the lack of food sources available to them (Breuil, 1997). In spit 1, reversely to all other splits, adult specimens of B. cavernarum dominates the bone assemblage and a lot of wings intentionally separated from the body of the bats, most of the time at the level of the humerus were present in the sediment. The anatomical distribution of the remains also indicates that the other body parts corresponding to these wings were absent from the bone assemblage. This kind of behavior (separation of the wings and body of the predated bats) has been observed on cats predating bats in Puerto Rico (Rodríguez-Durán et al., 2010) but spit 1 does not contain cat scats and this behavior is not backed by the observation made in our taphonomic analysis of the scats from spits 4 to 6 which indicate that bat wings were also consumed by the cats. These arguments make it difficult to attribute this strong concentration of wings in the spit 1 to predation by cats and the strong standardization of the behaviors we observed might also correspond to hunting by humans as bats are known to be occasionally consumed in Martinique (Lenoble, 2020; Negre, 1967) and that accumulations of bat wings in poaching sites have been observed by some of us in Guadeloupe. This hypothesis does however not explain the stronger representation of digestion marks on the bone material collected in the spit 1 which could be related to predation by another mammalian predator. In any case, this predation does not correspond to nowadays events as we removed the fresh guano before starting our excavation and during our visit to the cave, we saw no evidence of predation on bats other than the consumption by cockroaches and rats of individuals that felt naturally from the ceiling.
Regarding the dating of these different events, the occurrence of a plastic gun was produced since the ‘60s or ‘70s at the base of the guano deposit (facies “d” lens, just above the scree layer), combined with the obtained 14C date, allow us to estimate the minimum age of the base of the guano accumulation around 1960-1970 even if this layer as a whole may have formed during a much longer period (several hundred years). The layers containing the cat scats (spits 4–6) probably correspond to the situation observed by M. Breuil in 1994 (Breuil, 1997) but is now below 10–20 cm of additional sediment. These direct and hypothetical observations are coherent with a mean sedimentation rate of 1.6 cm per year in test pit 2. Considering this estimation and the few centimeters of fresh guano removed before the excavation, we estimate the formation of the upper layer of the stratigraphy to have formed around 5 years before our visit to the cave in 2017. The rate of sedimentation is lower in test pit-1, closer to the entrance of the cave, with the deposition of 0.85 cm of sediment per year. The maximal height of the cave is 3.5 m and its minimal height at the entrance is 1.5 m. In case the mean sedimentation rate generated by the bats stays the same, the site would be filled in 220 years and its entrance fully closed in 85 to 180 years. This means that the occupation of the site is not sustainable in the long run and that the colony will have to start looking for new shelters in the next decades.
Conservation and sanitary concerns raised by the future evolution of the colony
The observations from the literature indicate that the size of the B. cavernarum colony in the cave of Chancel Islet rose exponentially between 1979 and 2017. This is however in contradiction with the observations made in the historical record. Indeed, the speed of the guano deposition we estimated indicates that the cave has been densely occupied by this species since the formation of the “d” layer that we dated to around 1960-1970. We can thus hypothesize that the size of the colony shrank between 1960 and the first published observation of the colony in 1979 (Magnaval, 1984) which may be contemporaneous with the presence of cats in the cave even if this was only formally signaled in 1994 (Breuil, 1997). It is thus possible that the presence of cats in the cave led to a lesser frequentation of the site by B. cavernarum which also left some place for additional species to be present in the site as it is visible in the bone assemblage contemporaneous to the presence of cats as well as in the literature. The demise of the cats which exploited the cave seems to have reversed the situation to what it was previously with the presence of a massive monospecific colony of bats in the site that will inevitably lead to the filling and thus to the loss of the cave as bat roosting site in the next 100 to 200 years. In any case, we can observe that neither the predation by cats nor human seems to have had a significant long-term impact on the bat population size. Indeed, the past data provided by the paleoecological assemblages suggest that this site has been saturated by the colony for a long time and that the casual disturbances did not impact this global trend.
The stratigraphy of the Chancel Cave is very atypical as its sedimentation rate is several hundred times faster than the rate usually observed in the numerous cave paleontological deposits investigated in the Lesser Antilles in which B. cavernarum is a dominant bat species (Bochaton et al., 2015; Stoetzel et al., 2016; Stouvenot et al., 2014). The fact that such a sedimentation rate was never observed in the past record is a strong indication that the size of the colony of B. cavernarum and/or its concentration in the Chancel site is not a sustainable situation. This could reflect an imbalanced bat fauna reflecting human environmental modifications made on an island where synanthropic species dominate the now transformed environments. Several studies carried out in South American tropical forests show that environmental modification through deforestation and habitat fragmentation leads to a loss of species diversity in bat fauna and to the domination of modified environments by a few opportunistic species (Brosset et al., 1996; Cosson et al., 1999; Delaval & Charles-Dominique, 2006). In Martinique, such an imbalanced bat fauna is suggested by mist-net captures and acoustic detections showing that a few numbers of generalist species are overabundant in secondary environments (Barataud et al., 2017). Brachyphylla cavernarum is one of these species, along with the other Phyllostomid bat species, Artibeus jamaicensis, and the two insectivorous species Molossus molossus, and Pteronotus davyi. Moreover, Brachyphylla cavernarum is the most abundant species found in sampling stations (Barataud et al., 2017), notably in guava orchards where it is considered as a pest (Catzeflis et al., 2019). The fact that Brachyphylla cavernarum tends to become a dominant species in Lesser Antillean islands heavily impacted by humans is also evidenced in St. Lucia (Pedersen, Kwiecinski, et al., 2018) or Sint Eustatius (Pedersen, Larsen, et al., 2018). It is suggested as well by the fact that this species is one of the few species in the Lesser Antillean core bat fauna to occur on all the islands within its distribution range (Pedersen, Kwiecinski, et al., 2018). In addition, the beneficial effect of the human modification of the environment for this species is supported by the fossil record of Marie-Galante Island. This record shows that Brachyphylla cavernarum became a dominant species in the bat fauna during the Holocene and, even more so, in the second half of this period, i.e. when man was colonizing the archipelago. In the meantime, the species characterizing the pre-anthropic periods became very scarce or extirpated (Stoetzel et al. 2016). Brachyphylla cavernarum is a frugivorous bat (Nellis & Ehle, 1977), able to sustain on alternate forage (Pedersen et al., 2003), and to be occasionally or seasonally pollinivorous or insectivorous (Bond & Seaman, 1958; Lenoble et al., 2014; Pedersen et al., 1996), this dietary plasticity enabling it to cope with environmental vagaries (Pedersen, Kwiecinski, et al., 2018). In addition, the species benefits directly from the human transformation of the insular environments through the multiplication of the pioneer tree Cecropia schreberiana from which this bat obtains part of its subsistence (Barataud et al., 2017). It also strongly benefits from the introduction of fleshy fruits in the Lesser Antilles since the Amerindian times and their current concentration in the form of orchards (Picard & Catzeflis, 2013). All these conditions make Brachyphylla cavernarum a species that benefits from the competitive imbalance between the different bat species.
However, the saturation of the Chancel site could only indicate that the alternate best suitable roosts occupied by these bats are no longer available. It is noteworthy that all the medium and large caves in Martinique are occupied by animal colonies, birds, or bats, which demonstrates the saturation of the carrying capacity of the caves (Lenoble & Queffelec, 2016). The situation of the Chancel Cave saturated by B. cavernarum individuals can be encountered at a much smaller scale in man-made sites such as mines, wells, or old-building, as well as several important natural roosts of this species, hosting several thousand to tens of thousands individuals (Issartel & Jemin, 2017). The occupation of man-made roosting sites thus complements the small number of caves available on the island. The cave-nesting behavior of most West Indian bats is a factor of adaptation to the climatic and environmental hazards of the Lesser Antilles (Rodriguez-Duran, 2009) and these sites are thus crucial to maintain bat populations (Pedersen, Larsen, et al., 2018). The number of available roosting sites is thus likely to be the most important factor determining the size of the Brachyphylla cavernarum population in Martinique.
Whatever the explanation for the saturation of the Chancel site, sub-fossil data unambiguously demonstrates that the c.a. 250 000 bats of the Chancel Cave will have to find shelters others than this cave in a context of saturation of the existing roosts. The population of B. cavernarum roosting in Chancel corresponds to nearly half of the total population of this species in Martinique (Issartel & Jemin, 2017) and the unavailability of the Chancel Cave is very likely to have a major impact on the ecology of these bats in Martinique. This situation causes serious sanitary concerns as B. cavernarum is known to be able to roost near and inside human habitations and to be a vector of histoplasmosis in Martinique (Demoly, 2003; Leblanc & Issartel, 2008; Mouret, 1980), a disease associated to the fungus Histoplasma capsulatum, transmitted to human through the inhalation of the spores contained in bat guano. The occurrence of endemic cases of this zoonosis has been previously reported in Asia (Baker et al., 2019; Gopalakrishnan et al., 2012; Randhawa, 1970), Equatorial Africa, North and South America (Diaz, 2018), as well as in the Caribbean (Fincham, 1997; Tamsitt & Valdivieso, 1970), and La Martinique Island (Agossou et al., 2023; Demoly, 2003; Leblanc & Issartel, 2008; Magnaval, 1984; Mouret, 1980) where transmissions of this disease from bats to humans in anthropized areas occupied by B. cavernarum were also documented (Agossou et al., 2023; Minoza et al., 2016). Histoplasmosis is in most cases a benign disease for humans but its mortality rate can reach more than 6% in immunosuppressed patients (Assi et al., 2007; Kauffman et al., 1978). This disease as well as the potential threat caused by the presence of bat roosts in the area occupied by human populations cannot thus be ignored. In addition, bats can carry many other unknown zoonoses as may have been emphasized by the fact that several members of our excavation team presented, at the return of the fieldwork, symptoms of atypical lung infection, although we wore FFP3 masks during all the excavation, but were all negative to histoplasmosis serologies. The case of the Chancel Cave shows how anthropization phenomenon and conservation policies can impact the ecology of zoonoses which highlights the importance of integrative approaches to manage such diseases and prevent future outbreaks (Morand et al., 2019; Morand & Lajaunie, 2021; Schrag & Wiener, 1995).
CONCLUSION
Our investigation of the long-term evolution of the Chancel Cave shows how difficult it is to artificially keep the balance between species in environments disturbed by human activities. Paleobiological data alone are not enough to explain how the colony of B. cavernarum became so large and several hypotheses remain plausible. The most likely hypothesis, for now, is a combination of the feeding habits of B. cavernarum which favored the cultivated fruit easy to find in Martinique, the protected status of the species, and the absence of predators related to the conservation policies applied to the Chancel Islet. Independently of the causes, we unambiguously show that the roosting cave of the Chancel Islet runs to its demise as it cannot sustain such a large colony of bats in a near future. To avoid this situation, either control of the size of the bat colony should be set up or some work be conducted on the site to avoid its complete filling up. This last possibility would however be very difficult to put in place considering the poor accessibility of the site. The possible emergence of zoonosis from the Chancel colony should also not be overlooked and further work should be conducted on the pathogens these bats may carry. Finally, from a more global point of view, this study calls for further work regarding the often poorly investigated subfossil record to investigate biodiversity and sanitary questions to better understand past events and try to prevent arising and possibly elusive issues as it was the case for the Chancel Islet bat colony.
ACKNOWLEDGMENTS
The analysis of the material in the PACEA laboratory was conducted as a part of the ECSIT Project, conducted by the CNRS with financial support from the European PO-FEDER program (grant no. 2016-FED-503). The fieldwork operation has been funded by the Service Regional de l’Archéologie de Martinique.
The authors are grateful to the
Bally family who allowed us to conduct this work in the cave and on the Chancel
Islet. We are especially in debt to Michel Bally who assisted us during this
fieldwork and provided useful information regarding the history of the Chancel
Islet and its bats. We are also grateful to the researchers who provided
additional information regarding the fauna of Chancel namely Vincent Rufray,
Karl Questel, Chloé Rodriguez, and Baptiste Angin. The identification of the
plastic gun wads has been performed by the Cie Nle des Experts en Armes et
Munitions (Bernard Lucas and Pierre Laurent) and the preparation and
dissection of the cat scats by Christopher Martol. We also thank Benoit Bérard
and the Ouacabou association who assisted us in the financial management of
this project as well as the two anonymous reviewers who provided useful
comments on this manuscript. This work was conducted with the approbation of
the Service Regional de l’Archéologie de Martinique (permit n° 20170940001SRA) as
well as with that of the DEAL of Martinique. To finish, we dedicate this work
to the memory of F. Catzeflis who helped us in the preparation of this work.
This publication is the ISEM publication n° 2024-012.
REFERENCES
Adams, R. A. (2000). Wing ontogeny, shifting niche dimensions, and adaptive landscapes. In R. A. Adams & S. C. Pedersen (Eds.), Ontogeny, Functional Ecology, and Evolution of Bats (pp. 275–315). Cambridge University Press. https://doi.org/10.1017/CBO9780511541872.009
Agossou, M., Turmel, J.-M., Aline-Fardin, A., Venissac, N., & Desbois-Nogard, N. (2023). Acute pulmonary histoplasmosis of immunocompetent subjects from Martinique, Guadeloupe and French Guiana: A case series. BMC Pulmonary Medicine, 23(95), 1-8.
Assi, M. A., Sandid, M. S., Baddour, L. M., Roberts, G. D., & Walker, R. C. (2007). Systemic Histoplasmosis: A 15-Year Retrospective Institutional Review of 111 Patients. Medicine, 86(3), 162–169. https://doi.org/10.1097/md.0b013e3180679130
Baker, J., Setianingrum, F., Wahyuningsih, R., & Denning, D. W. (2019). Mapping histoplasmosis in South East Asia – implications for diagnosis in AIDS. Emerging Microbes & Infections, 8(1), 1139–1145. https://doi.org/10.1080/22221751.2019.1644539
Banerjee, A., Kulcsar, K., Misra, V., Frieman, M., & Mossman, K. (2019). Bats and Coronaviruses. Viruses, 11(1). https://doi.org/10.3390/v11010041
Barataud, M., Giosa, S., Issartel, G., Jemin, J., Lesty, M., & Fiard, J.-P. (2017). Forêts tropicales insulaires et chiroptères: Le cas de la Martinique (Petites Antilles – France). Le Vespère, 7, 411–457.
Barnosky, A. D., Hadly, E. A., Gonzalez, P., Head, J., Polly, P. D., Lawing, A. M., Eronen, J. T., Ackerly, D. D., Alex, K., Biber, E., Blois, J., Brashares, J., Ceballos, G., Davis, E., Dietl, G. P., Dirzo, R., Doremus, H., Fortelius, M., Greene, H. W., … Zhang, Z. (2017). Merging paleobiology with conservation biology to guide the future of terrestrial ecosystems. Science, 355(6325), eaah4787. https://doi.org/10.1126/science.aah4787
Bochaton, C., Grouard, S., Cornette, R., Ineich, I., Lenoble, A., Tresset, A., & Bailon, S. (2015). Fossil and subfossil herpetofauna from Cadet 2 Cave (Marie-Galante, Guadeloupe Islands, FWI): Evolution of an insular herpetofauna since the Late Pleistocene. Comptes Rendus Palevol, 14(2), 101–110.
Bochaton, C., Paradis, E., Bailon, S., Grouard, S., Ineich, I., Lenoble, A., Lorvelec, O., Tresset, A., & Boivin, N. (2021). Large-scale reptile extinctions following European colonization of the Guadeloupe Islands. Science Advances, 7(21), eabg2111. https://doi.org/10.1126/sciadv. abg2111
Boivin, N., & Crowther, A. (2021). Mobilizing the past to shape a better Anthropocene. Nature Ecology & Evolution, 1–12. https://doi.org/10.1038/s41559-020-01361-4
Bond, R. M., & Seaman, G. A. (1958). Notes on a Colony of Brachyphylla cavernarum. Journal of Mammalogy, 39(1), 150–151. https://doi.org/10.2307/1376623
Breuil, M. (1997). Les Reptiles, les Amphibiens et les Chauves-souris de l’îlet Chancel (Martinique). Direction Régionale de l’Environnement de Martinique-Association des Amis du Laboratoire des Reptiles et Amphibiens Muséum National d’Histoire Naturelle.
Brosset, A., Charles-Dominique, P., Cockle, A., Cosson, J.-F., & Masson, D. (1996). Bat communities and deforestation in French Guiana. Canadian Journal of Zoology, 74(11), 1974–1982.
Brunet-Rossinni, A. K., & Wilkinson, G. S. (2009). Methods for age estimation and the study of senescence in bats. In T. H. Kunz & T. S. Parsons (Eds.), Ecological and Behavioral Methods for the Study of Bats (pp. 315–325). (Johns Hopkins University Press).
Catzeflis, F., Issartel, G., & Jemin, J. (2018). New data on the bats (Chiroptera) of Martinique island (Lesser Antilles), with an emphasis on sexual dimorphism and sex ratios. Mammalia, 1–14.
Catzeflis, F., Issartel, G., & Jemin, J. (2019). New data on the bats of Martinique island (Lesser Antilles). Mammalia, 83(5), 501–514.
Cosson, J.-F., Pons, J.-F., & Masson, D. (1999). Effects of forest fragmentation on frugivorous and nectarivorous bats in French Guiana. Journal of Tropical Ecology, 15, 515–534.
Delaval, M., & Charles-Dominique, P. (2006). Edge effects on frugivorous and nectarivorous bat communities in a neotropical primary forest in French Guiana. Revue d’écologie (Terre Vie), 61(4), 343–352.
Demoly, A. (2003). Histoplasmose americaine disseminee avec atteinte oculaire. A propos d’une obervation. [M. D. Thesis]. Université Henry Poincaré.
Diaz, J. H. (2018). Environmental and Wilderness-Related Risk Factors for Histoplasmosis: More Than Bats in Caves. Wilderness & Environmental Medicine, 29(4), 531–540. https:// doi.org/10.1016/j.wem.2018.06.008
Dietl, G. P., & Flessa, K. W. (2011). Conservation paleobiology: Putting the dead to work. Trends in Ecology & Evolution, 26(1), 30–37. https://doi.org/10.1016/j.tree.2010.09.010
Dodson, P., & Wexlar, D. (1979). Taphonomic Investigations of Owl Pellets. Paleobiology, 5(3), 275–284.
Fincham, A. G. (1997). A word about histoplasmosis. In A. G. Fincham (Ed.), Jamaica underground (pp. 63–65). The press university of the West Indies.
Foster, G. W., Humphrey, S. R., & Humphrey, P. P. (1978). Survival Rate of Young Southeastern Brown Bats, Myotis austroriparius, in Florida. Journal of Mammalogy, 59(2), 299–304. https://doi.org/10.2307/1379913
Genoways, H. H., Larsen, R. J., Pedersen, S. C., Kwiecinski, G. G., & Larsen, P. A. (2012). Bats of Barbados. Chiroptera Neotropical, 17, 1029–1054.
Genoways, H. H., Pedersen, S. C., Larsen, P. A., Kwiecinski, G. G., & Huebschman, J. J. (2007). Bats of Saint Martin, French west indies/sint Maarten, Netherlands antilles. Mastozoología Neotropical, 14(2), 169–188.
Gopalakrishnan, R., Nambi, P., Ramasubramanian, V., Ghafur, A., & Parameswaran, A. (2012). Histoplasmosis in India: Truly uncommon or uncommonly recognised? The Journal of the Association of Physicians of India, 60, 25–28.
Hermanson, J. W., & Wilkins, K. T. (1986). Pre-Weaning Mortality in a Florida Maternity Roost of Myotis austroriparius and Tadarida brasiliensis. Journal of Mammalogy, 67(4), 751–754. https://doi.org/10.2307/1381140
Hill, C. A., & Forti, P. (1997). Cave minerals of the world. National Speleological Society.
Hughes, A. C., Tougeron, K., Martin, D. A., Menga, F., Rosado, B. H. P., Villasante, S., Madgulkar, S., Gonçalves, F., Geneletti, D., Diele-Viegas, L. M., Berger, S., Colla, S. R., de Andrade Kamimura, V., Caggiano, H., Melo, F., de Oliveira Dias, M. G., Kellner, E., & do Couto, E. V. (2023). Smaller human populations are neither a necessary nor sufficient condition for biodiversity conservation. Biological Conservation, 277, 109841. https://doi. org/10.1016/j.biocon.2022.109841
Issartel, G. (2000). Contribution à une meilleure conaissance et protection des Chiroptères de Martinique. SFEPM - Diren Martinique.
Issartel, G., & Jemin, J. (2017). Suivi des gîtes à chiroptères de Maritnique et mise en place de mesures de conservation. 2015-2016 [Rapport d’étude]. SFEPM.
Issartel, G., & Leblanc, F. (2004). Contribution à une meilleure conaissance et protection des Chiroptères de Martinique (mision mars 2004). SFEPM - Diren Martinique.
Kauffman, C. A., Israel, K. S., Smith, J. W., White, A. C., Schwarz, J., & Brooks, G. F. (1978). Histoplasmosis in immunosuppressed patients. The American Journal of Medicine, 64(6), 923–932. https://doi.org/10.1016/0002-9343(78)90445-X
Kemp, M. E., & Hadly, E. A. (2015). Extinction biases in Quaternary Caribbean lizards. Global Ecology and Biogeography, 24(11), 1281–1289. https://doi.org/10.1111/geb.12366
Kunz, T. H., & Anthony, E. L. P. (1982). Age Estimation and Post-Natal Growth in the Bat Myotis lucifugus. Journal of Mammalogy, 63(1), 23–32. https://doi.org/10.2307/1380667
Leblanc, F., & Issartel, G. (2008). Les risques liés à l’étude des chiroptères en outre-mer: Exemple de l’histoplasmose. Symbioses, (21), 63–64.
Lenoble, A. (2020). The Past Occurrence of the Guadeloupe Big-Eyed Bat Chiroderma improvisum Baker and Genoways, 1976 on Marie-Galante (French West Indies) with comments on Bat Remains from Pre-Columbian Sites in the Eastern Caribbean. Acta Chiropterologica, 21(2), 299–308. https://doi.org/10.3161/15081109ACC2019.21.2.005
Lenoble, A., Angin, B., Huchet, J.-B., & Royer, A. (2014). Seasonal Insectivory of the Antillean Fruit-Eating Bat (Brachyphylla cavernarum). Caribbean Journal of Science, 48(2–3), 127– 131. https://doi.org/10.18475/cjos.v48i3.a01
Lenoble, A., & Queffelec, A. (2016). Potentiel archéologique et paléontologique des cavités naturelles de Martinique: Rapport de prospection thématique. Service Régional de l’Archéologie, DAC Martinique.
Li, W., Shi, Z., Yu, M., Ren, W., Smith, C., Epstein, J. H., Wang, H., Crameri, G., Hu, Z., Zhang, H., Zhang, J., McEachern, J., Field, H., Daszak, P., Eaton, B. T., Zhang, S., & Wang, L.F. (2005). Bats Are Natural Reservoirs of SARS-Like Coronaviruses. Science, 310(5748), 676–679. https://doi.org/10.1126/science.1118391
Lyman, R. L. (2008). Quantitative paleozoology. Cambridge University Press.
Magnaval, J. F. (1984). Histoplasmosis and bats in Martinique (French West-Indies). Caribbean Journal of Science, 20(3–4), 109–112.
Mickleburgh, S. P., Hutson, A. M., & Racey, P. A. (2002). A review of the global conservation status of bats. Oryx, 36(1), 18–34. https://doi.org/10.1017/S0030605302000054
Minoza, A., Zecler, J., Miossec, C., Quist, D., Pierre-François, S., Deligny, C., Simon, S., Aznar, C., & Desbois, N. (2016). Cas groupés d’histoplasmoses à Histoplasma capsulatum var. capsulatum à la Martinique: Description des cas et enquête environnementale. Journal de Mycologie Médicale, 26(4), 377–384. https://doi.org/10.1016/j.mycmed.2016.09.001
Morand, S. (2020). Emerging diseases, livestock expansion and biodiversity loss are positively related at global scale. Biological Conservation, 248, 108707. https://doi.org/10.1016/j. biocon.2020.108707
Morand, S., Blasdell, K., Bordes, F., Buchy, P., Carcy, B., Chaisiri, K., Chaval, Y., Claude, J., Cosson, J.-F., Desquesnes, M., Jittapalapong, S., Jiyipong, T., Karnchanabanthoen, A., Pornpan, P., Rolain, J.-M., & Tran, A. (2019). Changing landscapes of Southeast Asia and rodent-borne diseases: Decreased diversity but increased transmission risks. Ecological Applications, 29(4), e01886. https://doi.org/10.1002/eap.1886
Morand, S., & Lajaunie, C. (2021). Outbreaks of Vector-Borne and Zoonotic Diseases Are Associated With Changes in Forest Cover and Oil Palm Expansion at Global Scale. Frontiers in Veterinary Science, 0. https://doi.org/10.3389/fvets.2021.661063 Mouret, C. (1980). Karst et pseudokarst de la Martinique. Spelunca, 1, 69–72.
Negre, A. (1967). Les Antilles à travers leur cuisine. Ozanne et Cie.
Nellis, D. W., & Ehle, C. P. (1977). Observations on the behavior of Brachyphylla cavernarum (Chiroptera) in Virgin Islands. 41(4), 403–410. https://doi.org/10.1515/mamm.1977.41.4.403
Nogué, S., de Nascimento, L., Froyd, C. A., Wilmshurst, J. M., de Boer, E. J., Coffey, E. E. D., Whittaker, R. J., Fernández-Palacios, J. M., & Willis, K. J. (2017). Island biodiversity conservation needs palaeoecology. Nature Ecology & Evolution, 1(7), 181. https://doi. org/10.1038/s41559-017-0181
Ourly, L. (2006). Conservation de l’iguane des Petites Antilles (Iguana delicatissima) en Martinique: Suivi des populations sur l’îlet Chancel et réintroduction sur l’îlet Ramiers [Mémoire de Master]. Université Paul Sabatier Toulouse III.
Parkinson, A. H. (2012). Dermestes maculatus and Periplaneta americana: Bone modification criteria and establishing their potential as climatic indicators [M. A. Thesis]. University of the Witwatersrand.
Pedersen, S. C., Genoways, H. H., Morton, M. N., Johnson, J. W., & Courts, S. E. (2003). Bats of Nevis, northern Lesser Antilles. Acta Chiropterologica, 5(2), 251–267.
Pedersen, S. C., Kwiecinski, G. G., Genoways, H. H., Larsen, R. J., Larsen, P. A., Phillips, C. J., & Baker, R. J. (2018). Bats of Saint Lucia, Lesser Antilles. Special Publications, Museum of Texas Tech University, 69, 1–61.
Pedersen, S. C., Larsen, P. A., Westra, S. A., van Norren, E., Overman, W., Kwiecinski, G. G., & Genoways, H. H. (2018). Bats of Sint Eustatius, Caribbean Netherlands. Occasional Papers of the Museum of Texas Tech University, 353, 1–24.
Pedersen, S., Genoways, H., & Freeman, P. (1996). Notes on Bats from Montserrat (Lesser Antilles) with Comments Concerning the Effects of Hurricane Hugo. Caribbean Jounal of Science, 32(2), 206 - 213.
Pelletier, M., Stoetzel, E., Cochard, D., & Lenoble, A. (2017). Sexual dimorphism in the pelvis of Antillean fruit-eating bat (Brachyphylla cavernarum) and its application to a fossil accumulation from the Lesser Antilles. Geobios, 50(4), 311–318. https://doi.org/10.1016/j. geobios.2017.06.001
Picard, R. (2017). Rapport d’expertise chiroptérologique: Sondage archéologique de la grotte de l’îlet Chancel (p. 12). Fredon Martinique.
Picard, R., & Catzeflis, F. (2013). Première étude des chauves-souris dans les goyaveraies de Martinique. In Vernier, J., Burac, M. (Eds.), Biodiversité Insulaire La Flore, La Faune et l’homme Dans Les Petites Antilles. (174–183). Université des Antilles et de la Guyane.
Randhawa, H. S. (1970). Occurrence of histoplasmosis in Asia. Mycopathologia et Mycologia Applicata, 41(1), 75–89. https://doi.org/10.1007/BF02051485
Reimer, P. J., Austin, W. E. N., Bard, E., Bayliss, A., Blackwell, P. G., Ramsey, C. B., Butzin, M., Cheng, H., Edwards, R. L., Friedrich, M., Grootes, P. M., Guilderson, T. P., Hajdas, I., Heaton, T. J., Hogg, A. G., Hughen, K. A., Kromer, B., Manning, S. W., Muscheler, R., … Talamo, S. (2020). The IntCal20 Northern Hemisphere Radiocarbon Age Calibration Curve (0–55 cal kBP). Radiocarbon, 62(4), 725–757. https://doi.org/10.1017/RDC.2020.41
Rodriguez-Duran, A. (2009). Bat assemblages in the West Indies: The role of caves. In T. H. Fleming & P. A. Racey (Eds.), Island Bats: Evolution, Ecology, and Conservation (pp. 265–280). University Press of Chicago.
Rodríguez-Durán, A., Pérez, J., Montalbán, M. A., & Sandoval, J. M. (2010). Predation by freeroaming cats on an insular population of bats. Acta Chiropterologica, 12(2), 359–362.
Schrag, S. J., & Wiener, P. (1995). Emerging infectious disease: What are the relative roles of ecology and evolution? Trends in Ecology & Evolution, 10(8), 319–324. https://doi. org/10.1016/s0169-5347(00)89118-1
Soto-Centeno, J. A., & Steadman, D. W. (2015). Fossils reject climate change as the cause of extinction of Caribbean bats. Scientific Reports, 5, 7971. https://doi.org/10.1038/srep07971
Stoetzel, E., Bochaton, C., Bailon, S., Cochard, D., Gala, M., & Laroulandie, V. (2021). MultiTaxa Neo-Taphonomic Analysis of Bone Remains from Barn Owl Pellets and CrossValidation of Observations: A Case Study from Dominica (Lesser Antilles). Quaternary, 4(4). https://doi.org/10.3390/quat4040038
Stoetzel, E., Royer, A., Cochard, D., & Lenoble, A. (2016). Late Quaternary changes in bat palaeobiodiversity and palaeobiogeography under climatic and anthropogenic pressure:
New insights from Marie-Galante, Lesser Antilles. Quaternary Science Reviews, 143, 150– 174. https://doi.org/10.1016/j.quascirev.2016.05.013
Stouvenot, C., Grouard, S., Bailon, S., Bonnissent, D., Lenoble, A., Serrand, N., & Sierpe, V. (2014). L’abri sous roche Cadet 3 (Marie-Galante): Un gisement à accumulations de faune et à vestiges archéologiques. In B. Bérard (Ed.), Actes du 24e Congrès de l’AIAC (pp. 126–140). IACA.
Swanepoel, P., & Genoways, H. H. (1983). Brachyphylla cavernarum. Mammalian Species, (205), 1–6. https://doi.org/10.2307/3503901
Tamsitt, J. R., & Valdivieso, D. (1970). Los murciélagos y la salud pública: Estudio con especial referencia a Puerto Rico. Boletín de La Oficina Sanitaria Panamericana (OSP), 69(2), 122–140.
Young, D., Benaglia, T., Chauveau, D., Hunter, D., Elmore, R., Hettmansperger, T., Thomas, H., & Xuan, F. (2016). mixtools: Tools for Analyzing Finite Mixture Models (1.0.4). https:// cran.r-project.org/web/packages/mixtools/index.html
Zinsstag, J., Schelling, E., Waltner-Toews, D., & Tanner, M. (2011). From “one medicine” to “one health” and systemic approaches to health and well-being. Preventive Veterinary Medicine, 101(3), 148–156. https://doi.org/10.1016/j.prevetmed.2010.07.003
Citation: Bochaton, C., Picard, R., Cochard, D., Conche, V., Lidour, K., & Lenoble, A. (2024). The recent history of an insular bat population reveals an environmental disequilibrium and conservation concerns.
Novitates Caribaea, (23), 22–50. https://doi.org/10.33800/nc.vi23.346