![]() Número 21, enero, 2023: 18–28
Número 21, enero, 2023: 18–28
ISSN versión impresa: 2071–9841 ISSN versión en línea: 2079–0139 https://doi.org/10.33800/nc.vi21.324
A NEW SPECIES OF EUPELTE (COPEPODA: HARPACTICOIDA: PELTIDIIDAE) FROM ANCHIALINE CAVES IN BERMUDA
Nueva especie de Eupelte (Copepoda: Harpacticoida: Peltidiidae) de cuevas anquihalinas de Bermuda
Carlos Varela1*, Thomas M. Iliffe2 and T. Chad Walter 3
![]() 1Institute of Environment, Department of Biological
Sciences, Florida International University, Florida, U.S.A.; https://orcid.org/0000-0003-3293-7562; cvare015@fiu.edu . 28 Cadena Drive, Galveston, Texas, 77554, U.S.A.; https://orcid.org/0000-0002-4342-5960; dr.tom.iliffe@gmail.com. 3Smithsonian Institution, Museum of Natural History,
U.S.A.; walterc@si.edu. *Corresponding author: cvare015@fiu.edu
1Institute of Environment, Department of Biological
Sciences, Florida International University, Florida, U.S.A.; https://orcid.org/0000-0003-3293-7562; cvare015@fiu.edu . 28 Cadena Drive, Galveston, Texas, 77554, U.S.A.; https://orcid.org/0000-0002-4342-5960; dr.tom.iliffe@gmail.com. 3Smithsonian Institution, Museum of Natural History,
U.S.A.; walterc@si.edu. *Corresponding author: cvare015@fiu.edu
[Received: August 3, 2022. Accepted: September 22, 2022]
ABSTRACT
A new anchialine species of Eupelte (Copepoda: Harpacticoida: Peltidiidae) is described from tidal pools and submerged passages in Bermuda caves. It is the sixth anchialine harpacticoid species known from Bermuda and the first anchialine member worldwide of the family Peltidiidae. The genus Eupelte currently contains 16 valid species, of which five have been found in the Atlantic Ocean, four in the Pacific Ocean, three in the Indian Ocean, two in the Mediterranean Sea, one in arctic waters and the last one in antarctic waters.
Keywords: subterranean estuary; Copepoda; stygiobiont; anchialine cave.
RESUMEN
Una nueva especie de Eupelte (Copepoda: Harpacticoida: Peltidiidae) es descrita de las galerías sumergidas de cuevas en Bermuda. Esta es la sexta especie anquialina de copépodo harpacticoide conocida para Bermuda, y la primera especie anquialina de la familia Peltidiidae. El género Eupelte presenta hasta el momento 16 especies válidas, de las cuales cinco especies han sido encontradas en el Océano Atlántico, cuatro en el Océano Pacifico, tres en el Océano Índico, dos en el Mar Mediterráneo, una en aguas árticas y la especie restante en aguas antárcticas.
Palabras clave: estuario subterráneo; Copepoda; estigobio; cueva anquialina.
INTRODUCTION
Harpacticoid copepods makeup a significant component of the world’s anchialine (saline or brackish waters with subterranean connections to the sea) cave fauna, with a few planktonic or symbiotic. They represent at least 15 species from eight genera and six families (Table I). The harpacticoid Family Superornatiremidae contains the most, with nine anchialine species (Huys, 1996; Jaume, 1997). These anchialine harpacticoids were found from submerged limestone and volcanic caves as well as connected groundwater aquifers. They include five species in Bermuda, three in the Balearic Islands, two each in Western Australia and North Vietnam, plus single species in Belize, Bahamas, and Canary Islands.
Bermuda represents an exceptional global hotspot for anchialine cave biodiversity (Iliffe & Calderón-Gutiérrez, 2021) with the Walsingham Cave System containing 78 described anchialine species of which 20 are copepods and five harpacticoids. It is not clear why Bermuda has the highest level of anchialine species, although possible explanations include the island’s isolated mid-Atlantic location, Pleistocene age limestone, and fluctuating sea levels which alternately submerged and exposed the anchialine caves.
Currently there are 16 valid species of the Genus Eupelte Claus, 1860: Eupelte acutispinis Zhang & Li, 1976 (China); Eupelte aurulenta Wells & Rao, 1987 (India); Eupelte beckleyaeHicks, 1982 (South Africa); Eupelte bicornis Claus, 1863 (North Sea); Eupelte cubensis Varela & Gomez, 2013 (Cuba); Eupelte gracilis Claus, 1860 (Mediterranean Sea); Eupelte hexaseta Hicks, 1982 (South Africa), Eupelte minuta (Ramirez, 1971) (Argentina); Eupelte oblonga Claus, 1886 (Mediterranean Sea); Eupelte purpurocincta (Norman, 1869) (Shetland Islands); Eupelte regalis Hicks, 1971 (New Zealand); Eupelte setacauda Monk, 1941 (California, USA); Eupelte simile (Monk, 1941) (California, USA); Eupelte tristanensis Wiborg, 1964 (Tristan da Cunha); Eupelte typica (Scott T., 1912); Eupelte villosa (Brady, 1910) (Tierra del Fuego); Eupelte oblivia Scott A., 1909 (South Orkneys) is currently accepted as Alteuthellopsis oblivia (Scott A., 1909), (Walter & Boxshall, 2021).
Table I. Worldwide anchialine Harpacticoida
![]()
|
Family |
Genus species |
Authors |
Location |
|
Ameiridae |
Nitokra humphreysi |
Karanovic & Pesce, 2002 |
W. Australia |
|
|
Nitokra vietnamensis |
Tran & Chang, 2012 |
North Vietnam |
|
Novocriniidae |
Novocrinia trifida |
Huys & Iliffe, 1998 |
Belize |
|
Peltidiidae |
Eupelte hughesi n. sp |
This paper |
Bermuda |
|
Rotundiclipeidae |
Rotundiclipeus canariensis |
Huys, 1988 |
Canary Islands |
|
Superornatiremidae |
Intercrusia problematica |
Huys, 1996 |
Bermuda |
|
|
Intercrusia garciai |
Jaume, 1997 |
Balearic Islands |
|
|
Neoechinophora daltonae |
Huys, 1996 |
Bermuda |
|
|
Neoechinophora fosshageni |
Huys, 1996 |
Bermuda |
|
|
Neoechinophora jaumei |
Huys, 1996 |
Bermuda |
|
|
Neoechinophora xoni |
Jaume, 1997 |
Balearic Islands |
|
|
Neoechinophora sp. |
Huys, 1996 |
Bahamas |
|
|
Superornatiremis mysticus |
Huys, 1996 |
Bermuda |
|
|
Superornatiremis mendai |
Jaume, 1997 |
Balearic Islands |
|
Tachidiidae |
Microarthridion thanhi |
Tran & Chang, 2012 |
North Vietnam |
|
Tetragonicipitidae |
Phyllopodopsyllus wellsi |
Karanovic, Pesce & Humphreys, 2001 |
W. Australia |
![]()
OBJECTIVES
- To describe a new species of copepod from the family Peltidiidae collected in a cave in Bermuda.
MATERIALS AND METHODS
Bermuda Cherry Pit Cave (32° 20.73’N, 64° 42.64’W) consists of a 30 m long by 12 m deep, water-filled fissure in Walsingham’s Idwal Hughes Nature Reserve (Fig. 1). Cherry Pit is adjacent and likely connected to the Palm Cave System, a complex series of submerged caverns and collapsed entrances (Iliffe & Calderón-Gutiérrez, 2021). It is located midway across the isthmus separating Harrington Sound and Castle Harbour. Tidal flushing of seawater moves through these caves such that salinity at deeper depths remains close to that of the surrounding ocean. The water level in the entrance pools from the Palm System and adjacent Cherry Pit fluctuate with the tides but with reduced amplitude and a two-hour delay from ocean tides. The surface water in the small entrance pool at Cherry Pit Cave has a salinity of 28.5, increasing to 35.4 at 1 m depth.
Collections from Cherry Pit Cave between 1982 and 1987 used a Deck Plankton Collector (Anderson, 2004) or scuba cave divers with 30 cm diameter, 93 µm mesh hand or plankton net (Iliffe, 2018). Copepoda were the most abundant organisms in these collections, numbering in the hundreds, followed in decreasing order by Ostracoda, Mollusca, Cumacea, Polychaeta, Amphipoda, and Chaetognatha. Among the Copepoda, Epacteriscus rapax Fosshagen, 1973 and Miostephos leamingtonensis Yeatman, 1980 were plentiful in collections from Cherry Pit Cave, along with a few specimens of a Metacalanus species (Fosshagen et al., 2001).
The abbreviations used in the text are: ae, aesthetasc; P1, Leg 1; P2, Leg 2; P3, Leg 3; P4, Leg 4, and P5, Leg 5.
RESULTS
Taxonomic Account
Order Harpacticoida
Family Peltidiidae Claus, 1860
Subfamily Peltidiinae Claus, 1860
Genus Eupelte Claus, 1860 (Figs. 2-4)
Eupelte hughesi sp. nov.
zoobank.org:pub:85E12DB7-5C4F-4952-B25D-E1E87FC9C847
Type material
Holotype. Non ovigerous female. 810 µm. BERMUDA. Collected in Cherry Pit Cave, 32° 20.73’N, 64° 42.64’W, 22.iii. 1987. 0-3 meters deep; col. T. Illife. National Museum of Natural History (USNM #1593336). Paratypes: One dissected non ovigerous female on slide (USNM #1593337); 16 non ovigerous females 805 µm (805 µm -810 µm) (USNM #261446).

Figure 1. Map of Bermuda. A) With cut-away; B) showing location of Cherry Pit Cave (#1), located in the isthmus of land between Harrington Sound and Castle Harbour. The rectangle indicates the approximate dimensions of the Walsingham Karst Region. Modified from Iliffe and Calderón Gutiérrez (2021).
Diagnosis (English). Eupelte hughesi n. sp. is unique with P1 endopod having six distal setae, small pointed projections on the lateral first third of the cephalothorax and four setae on the third article of the endopod of legs P2-P4. These six setae are only shared by E. hexaseta Hicks, 1982. Nevertheless, E. hughesi n. sp., differs from E. hexaseta in the small pointed projections on the lateral first third of the cephalothorax and the four setae on the third article of the endopod of legs P2-P4. In addition, E. hexaseta lacks the small pointed projections on the lateral first third of the cephalothorax and having five setae on the third article of the endopod in legs P2-P4. In additon, the female P5 terminal spines are thin and fine, whereas in E. hexaseta the are stout and hirsute on all spines.
Diagnosis (Spanish). Eupelte hughesi, n. sp. es único con el endópodo de P1 con seis setas distales, pequeñas proyecciones puntiagudas en el primer tercio lateral del cefalotórax y cuatro setas en el artejo 3 del endópodo de las patas P2-P4. Estas seis setas en el endópodo de P1, solo las comparte E. hexaseta Hicks, 1982. Sin embargo, E. hughesi n. sp., se diferencia de E. hexaseta en las pequeñas proyecciones puntiagudas en el primer tercio lateral del cefalotórax y las cuatro setas en el tercer artículo del endópodo de las patas P2-P4. Además, E. hexaseta carece de las pequeñas proyecciones puntiagudas en el primer tercio lateral del cefalotórax y tiene cinco setas en el artejo 3 del endópodo en las patas P2-P4. Además, las espinas terminales de P5 de la hembra son delgadas y finas, mientras que en E. hexaseta estas espinas son robustas e hirsutas.
DESCRIPTION
Female
Total length. 810 µm. Prosome: Urosome ratio (590 µm: 320 µm), including rostrum and caudal rami and width 460 µm at the widest point of prosome. Body flattened dorsoventrally, weakly arched along the midline, ovoid in shape, without a complex pattern of chitinous thickenings cephalothorax with four somites. Posterior dorsal margins on each somite finely serrated and with a row of tactile hairs, and each lateral corner sharply produced posteriorly, with several lateral setae on each. Rostrum wide and trapezoid, not defined at the dorsal surface, and the tip bends downwards and forwards (Fig. 2D). Urosome consists of four somites. Genital double somite is rectangular and wide, with two large posteriorly directed protrustions at midlength. Second abdominal somite corners spinelike posteriorly directed, third somite is small and narrow and anal somite is almost as wide as the third abdominal somite. Each segment with lateral setae rows and posterior margins finely serrated (Fig. 2A). Caudal rami slightly longer than wide with oblique posterior margin terminating with one long and three short setae, one mediolateral plumose seta and two subdistial surface setae on the back side, medial and lateral margins with spinule rows (Fig. 2E).
Antennule. 9-segmented, first article with fine spinules along distolateral surface. Articles four and nine with 4+ae. The setae formula is as follows: 1; 7; 5; 3+ae; 2; 2; 2; 2; 6+ae (Fig. 2B).
Antenna. Rectangular basipod, twice as long as wide, with distolateral seta, a small 2-segmented exopod, first with one terminal seta as long as the basipodite, second segment small rounded with three terminal setae. Endopod 2-segmented, rectangular with medial seta, second segment elongated with two distal setae, and five terminal setae, five are geniculate and one small and simple (Fig. 2C).
Maxillule. With a long narrow and distally directed praecoxapod segment, with seven distomedial strong setae, one terminal strong spine and two setae. Coxopod small with three setae and basipodite with five setae and an unguiform spine. Endopod represented by one seta and exopod with three setae (Fig. 3A).
Maxilla. Praecoxapod flattened and rectangular with three endites, proximal one with one simple and one finely spinulose setae, the medial and distal with two and three, setae respectively. Endopodite reduced and represented by three setae. Basal endite narrow bearing one strong unguiform seta and one simple setae (Fig. 3B).
Mandible. Thin and elongated precoxa with five strong distal spines, three are bidentate and one tridentate, a simple and spinulous seta at internal bulge. Endopod with five spiny setae and small narrow exopod with three simple terminal setae (Fig. 3C).
Maxilliped. Terminally prehensile, slender, basipod narrow elongated with two subterminal medial setae. Exopod 2-segmented, first segment ovoid inflated proximally and slightly concave along medial surface, medial margin with typically 11 stout spines, second segment distally elongated as curved pointed claw with a tiny basal seta (Fig. 2F).
Leg1 with elongated rectangular coxopod. Basipod with a long proximal and a large mediolateral seta with spinules along outer margin. Exopod 3-segmented, the second is the longest of the three with two setae, the first with one seta, the third short, almost as wide as long with four curved strong spines. Endopod 2-segmented, the first with one medial seta and second with six terminal setae (Fig. 3D).
Legs 2-4 with a pair of 3-segmented exopods and endopods swimming legs. The setae and spine formula are as follows: Coxa no spines or spinules. Basis with one terminal setae. Exopod I-1:0; II-1:1; III-3:5 and Endopod I-0:1; II-0:2; III-0:5 (Figs. 3E , 4A, 4B).
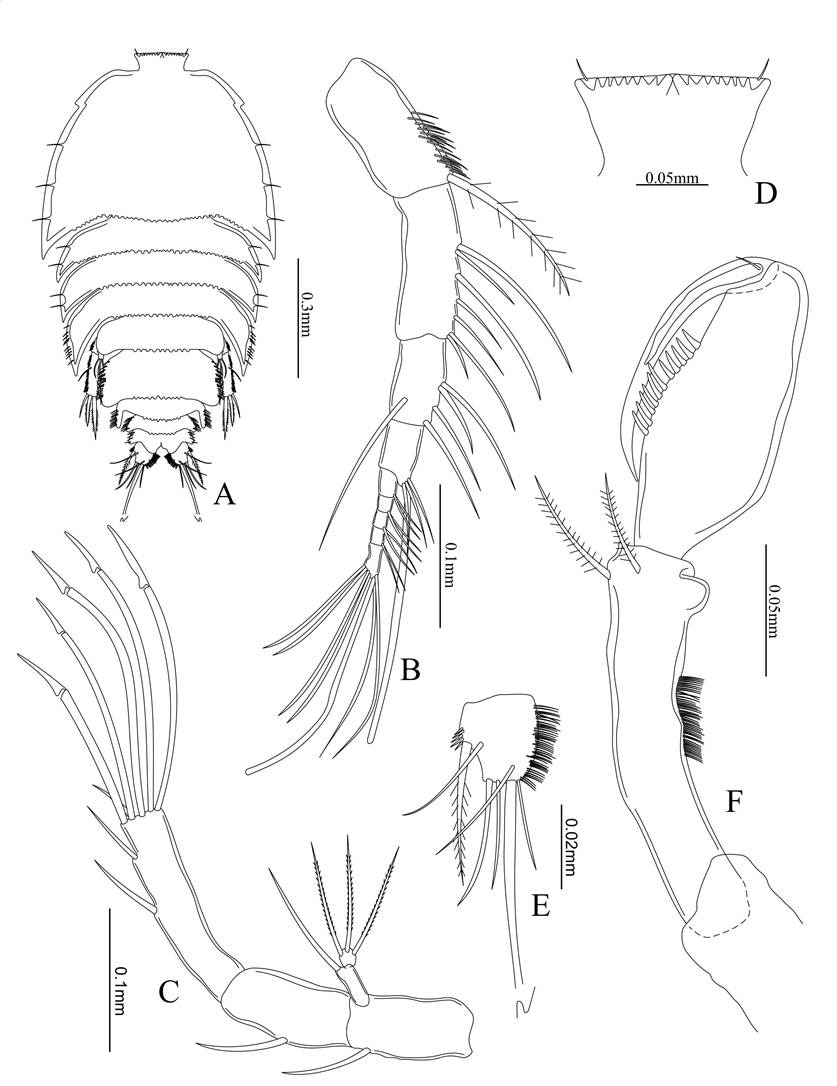
Figure 2. Eupelte hughesi sp. nov., female holotype. A) Dorsal view; B) antennule; C) antenna; D) rostrum; E) caudal ramus; F) maxilliped.
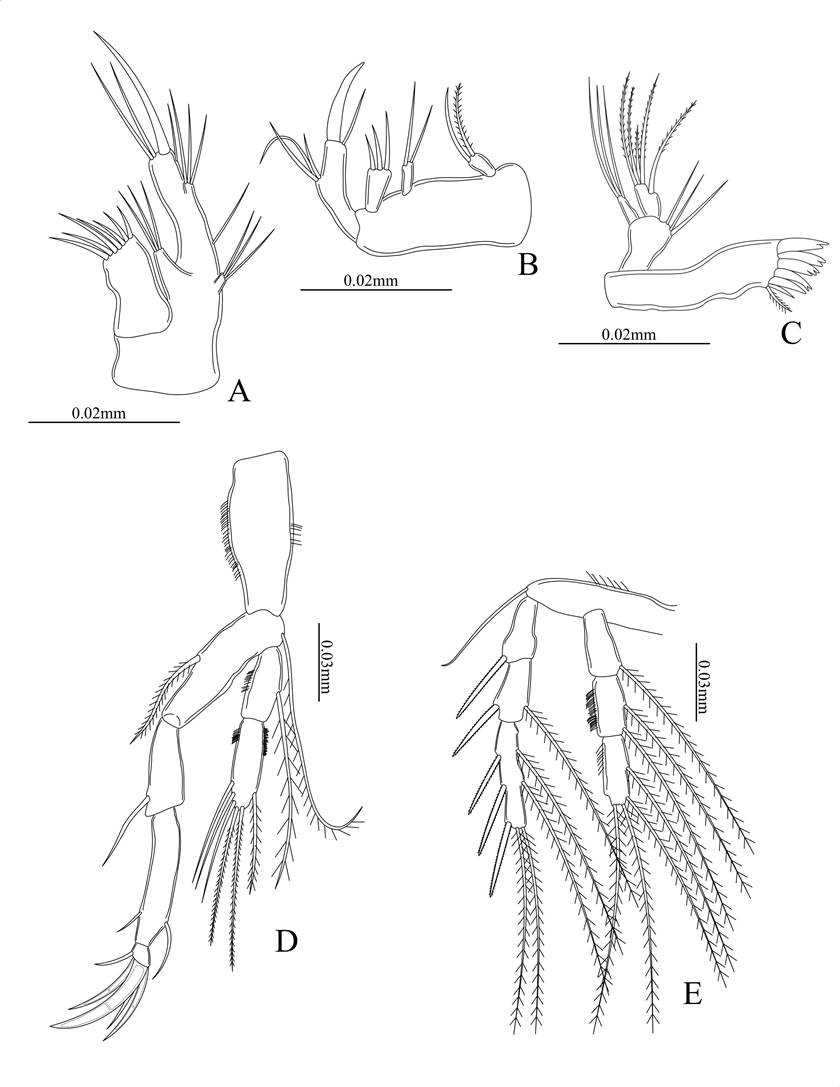
Figure 3. Eupelte hughesi sp. nov., female holotype. A) Maxillule; B) maxila; C) mandible; D) Leg 1; E) leg 2.

Figure 4. Eupelte hughesi sp. nov., female holotype. A) Leg 3; B) leg 4; C) leg 5.
The seta and spine formula are as follows:
Leg Exopod Endopod
P2 0: 1: 223 1: 2: 220
P3 0: 1: 323 1: 2: 220
P4 0: 1: 323 1: 2: 220
Leg 5 with baseoendopod bilobed. Outer lobe with one seta and a row of spines on the outer margin. Inner lobe short, inserted on anterior surface of baseoendopod, with a large simple seta near its base and three longer setae distally. Exopod elongate, with two outer, two inner and one larger terminal hairy setae. Outer margin with minute spines (Fig. 4C).
Male: unknown.
Etymology. Eupelte hughesi is named in recognition of the Idwal Hughes Nature Reserve, the type locality for this species.
Remarks. Eupelte hughesi n. sp. presents a combination of two characters that are unique in the genus Eupelte. The P1 endopod having six distal setae only shared by E. hexaseta Hicks, 1982 and the presence of small pointed projections on the lateral first third of the cephalothorax, character only shared by E. gracilis Claus, 1860. Eupelte hughesi n. sp. has P1 endopod having six distal setae, small pointed projections on the lateral first third of the cephalothorax and four setae on the third article of the endopod of legs P2-P4. These six distal setae on P1 endopod are only shared by E. hexaseta. Nevertheless E. hughesi n. sp., differs from E. hexaseta in the small pointed projections on the lateral first third of the cephalothorax and the four setae on the third article of the endopod of legs P2-P4. In addition, E. hexaseta lacks the small pointed projections on the lateral first third of the cephalothorax and having five setae on the third article of the endopod in legs P2-P4. In additon, the female P5 terminal spines are thin and fine, whereas in E. hexaseta the are stout and hirsute on all spines. Eupelte gracilis Claus, 1860, present the small pointed projections on the lateral first third of the cephalotorax. This species has been redescribed on several occasions but some of the diagnostic characters of those redescriptions sometimes don't match, this suggests that it may be a species complex (Varela & Gomez, 2013). Nevertheless, E. hughesi n. sp. presents the P1 endopod having six distal setae and the leg 5 of the female present 5 setae on the basoendopod and 6 setae on the exopod (5:6) and E. gracilis present the P1 endopod having four distal setae and a different armature in the leg 5 of the female.These differences presented here are sufficient to justify the recognition of a new species.
ACKNOWLEDGMENTS
We thank the Idwal Hughes Nature Reserve and the Walsingham Nature Reserve for their assistance and support in permitting access to the collection site. Support was also provided by the Bermuda Biological Station for Research and the Bermuda Aquarium, Museum and Zoo. Members of the Bermuda Cave Diving Association assisted with the cave diving associated with this research.
REFERENCES
Anderson, G. (2004, Jul 8). Tools of the Oceanographer: Sampling Equipment. MarineBio.net. Retrieved Apr. 4, 2022, from https://www.marinebio.net/marinescience/01intro/tosamp.htm
Brady, G. S. (1910). Die marinen Copepoden der Deutschen Südpolar Expedition 1901-1903. I, Ueber die Copepoden der Stämme Harpacticoida, Cyclopoida, Notodelphyoida und Caligoida. Deutsch. Südpolar Expedition, 1901-1903, 11. Zoologie, 3, 497–594.
Claus, C. (1860). Beiträge zur Kenntis der Entomostraken. 1. Ueber Saphirinen. Marburg, Germany. Erstes Heft, 1, 1–28.
Claus, C. (1863). Die Frei Lebenden Copepoden: Mit Besonderer Berücksichtigung Der Fauna Deutschlands, Der Nordsee Und Des Mittelmeeres. Verlag von Wilhelm Engel. https://doi. org/10.5962/bhl.title.58676
Claus, C. (1886). Neue Beiträge zur Morphologie der Crustaceen. Arbeiten aus dem Zoologischen Institute der Universität Wien und der Zoologischen Station in Triest. 6, 1–108, 7pls. [for 1885].
Fosshagen, A. (1973). A new genus and species of bottom living calanoid (Copepoda) from Florida and Colombia. Sarsia, 52(1), 145–154. https://doi.org/10.1080/00364827.1973.10 411237
Fosshagen, A., Boxshall, G., & Iliffe, T. (2001). The Epacteriscidae, a cave-living family of calanoid copepods. Sarsia, 86(4–5), 245–318. https://doi.org/10.1080/00364827.2001.10 425520
Hicks, G. R. (1971). Some littoral harpacticoid copepods, including five new species, from Wellington, New Zealand. New Zealand Journal of Marine and Freshwater Research, 5(1), 86–119. https://doi.org/10.1080/00288330.1971.9515371
Hicks, G. R. (1982). Porcellidiidae and Peltidiidae (Copepoda: Harpacticoida) from the marine algae of St Croix Island, Algoa Bay, South Africa. Zoological Journal of the Linnean Society, 75(1), 49–90.
Huys, R. (1988). Stygofauna of the Canary Islands: 10. Rotundiclipeidae Fam. Nov. (Copepoda, Harpacticoida) from an anchihaline cave on Tenerife, Canary Islands. Stygologia, 4(1), 42–63.
Huys, R. (1996). Superornatiremidae fam. nov. (Copepoda: Harpacticoida): An enigmatic family from North Atlantic anchihaline caves. Scientia Marina, 60(4), 497–542.
Huys, R. & Iliffe, T. M. (1998). Novocriniidae, a new family of harpacticoid copepods from anchihaline caves in Belize. Zoologica Scripta, 27(1), 1–15.
Iliffe, T. M. (2018). Collecting and processing crustaceans from anchialine and marine caves. Journal of Crustacean Biology, 38(3), 374–379.
Iliffe, T. M. & Calderón-Gutiérrez, F. (2021). Bermuda’s Walsingham Caves: A global hotspot for anchialine stygobionts. Diversity, 13(8), 352. https://doi.org/10.3390/d13080352
Jaume, D. (1997). First record of Superornatiremidae (Copepoda: Harpacticoida) from Mediterranean waters, with description of three new species from Balearic anchihaline caves. Scientia Marina, 61(2), 131–152.
Karanovic, T. & Pesce, G. L. (2002). Copepods from ground waters of Western Australia, VII. Nitokra humphreysi sp. nov. (Crustacea: Copepoda: Harpacticoida). Hydrobiologia, 470(1), 5–12.
Karanovic, T., Pesce, G. L., & Humphreys, W. F. (2001). Copepods from ground waters of Western Australia, V. Phyllopodopsyllus wellsi sp. novo (Crustacea: Copepoda: Harpacticoida) with a key to world species. Records of the Western Australian Museum, 20, 333–344.
Monk, C. R. (1941). Marine harpacticoid copepods from California. Transactions of the American Microscopical Society, 60, 75–99.
Norman, A. M. (1869). Last report on dredging among the Shetland Isles. Part 2. On the Crustacea, Tunicata, Polyzoa, Echinodermata, Actinozoa, Hydrozoa, and Porifera. In Association for Advancement of Science (Ed.), Report of the thirty-eighth meeting of the British Association for the Advancement of Science; held in Norwich in August 1868 (pp. 247–336). Murray London.
Ramírez, F. C. (1971). Paralteutha minuta, una nueva especie de Copépodos (Harpacticoida, Peltidiidae) hallado en aguas costeras de Mar del Plata, Argentina. Revista del Museo de La Plata, Nueva Serie 11, Zoología, 99, 115–119.
Scott, A. (1909). The Copepoda of the Siboga Expedition. Part I. Free-swimming, littoral and semi-parasitic Copepoda. Siboga Expeditie, 29, 1–323.
Scott, T. (1912). The Entomostraca of the Scottish National Antarctic Expedition, 1902-1904. Transactions of the Royal Society of Edinburgh, 48(3), 521–599.
Tran, D. L. & Chang, C. Y. (2012). Two new species of harpacticoid copepods from anchialine caves in karst area of North Vietnam. Animal Cells and Systems, 16(1), 57–68.
Varela, C. & Gómez, S. (2013). Dos nuevas especies de la familia Peltidiidae Boeck, 1873 (Copepoda: Harpacticoida) de Cuba. Novitates Caribaea, (6), 51–62.
Walter, T. C. & Boxshall, G. (2021). World of Copepods Database. Eupelte Claus, 1860.
Retrieved February 12, 2022, from http://www.marinespecies.org/copepoda/aphia. php?p=taxdetails&id=115428.
Wells, J. B. J. & Rao, G. C. (1987). Littoral Harpacticoida (Crustacea: Copepoda) from Andaman and Nicobar Islands. Memoirs of the Zoological Survey of India, 16(4), 1–385.
Wiborg, K. F. (1964). Marine copepods of Tristan da Cunha. Results of the Norwegian Scientific Expedition to Tristan da Cunha, 51, 1–44.
Yeatman, H. C. (1980). Miostephos leamingtonensis, a new species of copepod from Bermuda. Journal of the Tennessee Academy of Science, 55(1), 22–21.
Zhang, C. Z. & Li, Z. Y. (1976). Harpacticoida (Copepoda, Crustacea) from Xisha Island of Guangdong Province, China. Acta Zoologica Sinica, 22(1), 66–70.
Citation: Varela, C., Iliffe, T. M., & Walter, T. C. (2023). A new species of Eupelte (Copepoda: Harpacticoida: Pezltidiidae) from Anchialine Caves in Bermuda. Novitates Caribaea, (21), 18–28.doi.org/10.33800/nc.vi21.324
