![]() Número 21, enero, 2023: 1–17
Número 21, enero, 2023: 1–17
ISSN versión impresa: 2071–9841 ISSN versión en línea: 2079–0139 https://doi.org/10.33800/nc.vi21.323
A REMARKABLE NEW SNAKE OF THE GENUS TROPIDOPHIS
(SQUAMATA: TROPIDOPHIIDAE) FROM SOUTHERN HISPANIOLA
Una notable nueva serpiente del género Tropidophis
(Squamata: Tropidophiidae) del sur de la Hispaniola
Miguel A. Landestoy T.
Escuela
de Biología, Universidad Autónoma de Santo Domingo; República Dominicana. Av. Alma
Mater, Distrito Nacional; ![]() https://orcid.org/0000-0002-5072-5769; hispanioland@gmail.com
https://orcid.org/0000-0002-5072-5769; hispanioland@gmail.com
[Received: October 17, 2022. Accepted: October 31, 2022]
ABSTRACT
A new species of Tropidophis is described from the dry forest of the Barahona Peninsula, southwestern Dominican Republic, on the Caribbean island of Hispaniola. There, the new species is parapatric with T. haetianus, the only previously known Tropidophis on Hispaniola, but exhibits striking differences in scalation (much higher number of ventral scales), in other structural morphological traits (head and body proportions), and in dorsal and ventral coloration and pattern (e.g., fewer spot rows, and a patternless head and venter, etc.). The locality of this new species lies within an area where other vertebrate species have been recently discovered, underscoring the growing appreciation of the Barahona Peninsula as a diversity hotspot. Yet, this region also remains poorly studied and it is also highly imperiled due to ongoing anthropogenic change, justifying more conservation efforts.
Keywords: Caribbean Islands; dwarf boas; Barahona Entrapment.
RESUMEN
Se describe una nueva especie de Tropidophis del bosque seco del procurrente de Barahona, en el suroeste de la República Dominicana, en la isla caribeña de la Hispaniola. Allí, la nueva especie es parapátrica a T. haetianus, la única especie previamente conocida en la Hispaniola, pero exhibe notables diferencias en escamación (mucho mayor número de escamas ventrales), en otros rasgos estructurales morfológicos (proporción de cabeza y cuerpo) y en coloración y patrón dorsal y ventral (e.g., menos filas de manchas, y la cabeza y vientre sin patrón, etc.). La localidad de esta nueva especie yace en un área en donde varias especies de vertebrados han sido descubiertas recientemente, destacando la importancia del procurrente de Barahona como un punto caliente de biodiversidad. Sin embargo, esta región también permanece poco estudiada y en alto riesgo debido al cambio antropogénico en curso, lo que justifica mayores esfuerzos de conservación.
Palabras clave: islas del Caribe; boas enanas; entrampamiento de Barahona.
INTRODUCTION
The Neotropical snake genus Tropidophis (Bibron in Cocteau & Bibron, 1843) is currently comprised of 33 recognized species (Uetz et al., 2022), with 28 occurring on several Caribbean islands (sensu Díaz & Cádiz, 2020; Hedges et al., 2019) and five species found in mainland South America (Cursio et al., 2012; Díaz & Cádiz, 2020). More than half of the species (17) occur in the Cuban Archipelago alone, where several species co-occur (Díaz & Cádiz, 2020; Hedges, 2002), and three species occur in Jamaica (Hedges, 2002; 2022; Uetz et al., 2022). The remaining species are restricted to one-species islands (Lucayan Archipelago, Cayman Islands, Navassa, and Hispaniola). Despite the large size of Hispaniola (~76,000 km2), only one species—Tropidophis haetianus Cope—is known from this island (Cochran, 1941; Schwartz & Marsh, 1960; Stull, 1928), where it can be found in several habitat types across a wide geographic range, including the offshore Gonave and Tortue islands (Hedges, 2022; Henderson et al., 2021; Henderson & Powell, 2009). Some phenotypic discontinuity has been recognized, corresponding to several subspecies: T. h. haetianus is distributed islandwide, T. h. hemerus Schwartz restricted to the eastern end of the Dominican Republic, and T. h. tiburonensis Schwartz restricted to the western half of the Tiburon Peninsula (Hedges, 2002; Schwartz, 1975; Schwartz & Henderson, 1991; Schwartz & Marsh, 1960). Non-Hispaniolan populations previously assigned to T. haetianus have either been described as new species (T. hendersoni Hedges & Garrido, 2002) or elevated to specific status (T. jamaicensis Stull, T. stejnegeri Grant and T. stullae Grant; Hedges, 2002). A comprehensive revision is needed to assess the actual taxonomic status of T. haetianus throughout its current range (Hedges, 2002), but no specieslevel differentiation has been found for this species to date, retaining Hispaniola as the largest one-species island for this diverse snake radiation. The most geographically proximate relative is T. bucculentus (Cope), a (possibly extinct) species from Navassa Island (ca. 55 km W of mainland Hispaniola). Nevertheless, it is more closely related to the Cuban species T. melanurus (Schlegel) (Hedges, 2002).
Tropidophis snakes often display cryptic coloration and can be relatively difficult to find, which has led to the description of several species based on a single specimen (Díaz & Cádiz, 2020; Hedges & Garrido, 1999; 2002; Hedges et al., 1999). This might be the reason for which other species of Tropidophis have never been found in Hispaniola. While working in the field our team unexpectedly found two specimens of Tropidophis so strikingly different from the Hispaniolan representative, and from the rest of its congeners, that it undoubtedly constitutes an undescribed species. Herein I describe a second Tropidophis species known to Hispaniola, 143 years after the description of T. haetianus. This new species is distinct based on external phenotypic traits, specifically its strikingly different structure (shape, morphometrics, meristics) and color (coloration and pattern). The specimens were found in an area of ongoing deforestation close to the border with Haiti and, with massive tourism development ahead, a formal description is urged to facilitate conservation assessment and plans.
OBJECTIVES
- To describe a new species of snake of the genus Tropidophis from the island of Hispaniola.
MATERIALS AND METHODS
I compared the new species with its congeners by examining several morphological variables. Data from the new species and from 26 Tropidophis haetianus specimens were taken from recently collected individuals. For other specimens of T. haetianus and the remaining species I obtained morphological data from Hedges (2002), and from other previously published literature (Table I). For the morphological description, the terminology and methods follow Schwartz and Marsh (1960) and Hedges (2002). I measured eight linear morphometric characters using digital calipers (precision ±0.01mm) while viewing the specimen under a Motic K-400 stereoscope. Some of the characters (defined below) were measured from high resolution images taken from freshly euthanized specimens, since they exhibited some degree of altered shape due to preservation; measurements in this case were made with a size scale using the Straight tool from the ImageJ software (http://rsb.info.nih.gov/ij/). Similarly, characters from some specimens were discarded due the poor condition of such (roadkill, dehydrated, or mutilated specimens). Fourteen meristic characters were also scored, although only the four most relevant are displayed in Table I.
Abbreviations are as follows: snout-vent-length (SVL, measured from the tip of the snout to the vent), head length (HL, from the tip of snout to the jaw angle), head width (HW, from the widest part of head), neck width (NW, from behind the occiput), snout length (SNL, from tip of snout to anterior corner of eye), eye diameter (ED, horizontal distance across the eye), internarial distance (IND, closest distance between nostrils), interorbital distance (IOD, closest distance between eyes dorsally, which is the intersection between the supraorbital and preocular scales). HW and NW were measured when warranted (see above), and when a scale was not available in the photo, the IOD was set as a reference measurement. Proportions that resulted in ratio calculations were obtained from the aforementioned variables (e.g., ED/HW). MALT (field tag series, Miguel A. Landestoy T., Dominican Republic), MPM VZP (photographic vouchers deposited in the collection of the Milwaukee Public Museum), MNHNSD (Museo Nacional de Historia Natural “Prof. Eugenio de Jesús Marcano”, Santo Domingo, Dominican Republic).
RESULTS
Tropidophis leonae sp. nov.
Jaragua Golden Trope
Figures 1, 3-A, 3-B, 4-A, 5-A and 7.
urn:lsid:zoobank.org:act:FA9CDD65-2330-4366-8D09-42614B12D10B
Holotype (Figs. 1, 2, 3A and 3B). MNHNSD 23.3952 (MALT 723); MPM VZP1072; a female (mass in life 18.3 g) collected in the limestone foothills of Loma Las Trincheras, Paso Sena, 4 km N of Pedernales, Pedernales Province, on 4 November 2020 by Miguel A. Landestoy T. and Yimell Corona.
Paratype. MNHNSD 23.3951 (MALT 226); MPM VZP1071; a female (mass in life 19.0 g) from the same locality and collectors as the holotype, on 18 November 2018.
Diagnosis. A medium-sized (SVL 362–389 mm) species of Tropidophis of slender habitus (body somewhat laterally compressed), a distinctive neck and long snout, small eyes, high ventral scale count, a pale dorsal coloration of yellow-tan to light tan-brown with a dorsal pattern of only four brown spot rows, the middorsal blotches at times in contact or fused, and the lateral spot row much smaller and fainter; a pale yellow venter, and lacking a ventral and head pattern.
Tropidophis leonae sp. nov. requires close comparison with the only other Hispaniolan congener, T. haetianus (Figs. 3–5). It differs from parapatric T. haetianus by the following characters (Table I): 1) the smaller size (SVL to 389 vs. to 552 mm in T. haetianus); 2) a higher ventral scale count (216–217 vs. 170–194); 3) the slender and somewhat laterally compressed habitus (vs. robust and cylindrical in T. haetianus); 4) the more distinctive neck (HW/NW 1.6 vs. 1.2–1.5); 5) the longer snout (SNL/HL 0.34 vs. 0.30–0.34); 6) the proportionately smaller eyes (ED/HW 0.20 vs. 0.21–0.30, ED/HL 0.12 vs. 0.13–0.16, ED/SNL 0.36 vs. 0.41–0.50, ED/IND 0.68–0.72 vs. 0.79–1.04); 7) narrower interorbital distance (IOD/HW 0.46–0.47 vs. 0.48–0.60, IOD/HL 0.28 vs. 0.30–0.36); 8) the lower spot row count (4 vs. 8–10, rarely 6 in T. haetianus); 9) spot color in life (milk chocolate brown in T. leonae sp. nov. vs. shades of olive-green freckled with black in T. haetianus); 10) the small and faint lateral spot row (vs. large and well defined spots in T. haetianus; Fig. 3); 11) dorsal area of the head patternless, nearly uniformly orange-tan (vs. head darker than dorsum, often with dark figures); 12) lateral area of the head nearly uniformly tan-brown, with yellow suffusions (vs. a dark olive-brown to blackish mask or eye stripe contrasting with very pale labials and throat; Fig. 5); 13) lacking a ventral pattern (small, irregular yellow-tan smudges limited to edges of some scales vs. large and very dark spots; Fig. 4). The other geographically proximate species to T. leonae sp. nov. is T. bucculentus (from Navassa Island), from which this new species differs by its smaller size (SVL to 389 vs. to 596 mm), a slender habitus (vs. robust), the higher number of ventral scales (216–217 vs. 183–186 in T. bucculentus), the more distinctive neck (HW/NW 1.6 vs. 1.5), smooth dorsal scales (vs. keeled), and in having both fewer spot rows (4 vs. 6) and body spots (43–46 vs. 48–54).
Other Caribbean species that warrant comparison with Tropidophis leonae sp. nov. in which overlapping in the ventral scale count occurs, are discarded as follows: T. leonae sp. nov. is distinguished from T. wrighti by virtue of a smaller size (SVL to 389 vs. to 488 mm in T. wrighti; Domínguez et al., 2005), a less distinctive neck (HW/NW 1.6 vs. 1.8–2.2), proportionately smaller eyes (ED/HW 0.20 vs. 0.32–0.34), and more body spots (43–46 vs. 21–37); from T. semicinctus it differs in proportionately smaller eyes (ED/HW 0.20 vs. 0.30–0.34), and both in a higher number of spot rows (4 vs. 2) and body spots (43–46 vs. 17–26); from T. melanurus it differs in the much smaller size (SVL to 389 vs. >1000 mm; Díaz & Cádiz, 2020; Rodríguez-Cabrera et al., 2021), proportionately smaller eyes (ED/HW 0.20 vs. 0.21–0.26), and in the condition of the dorsal scales (smooth vs. keeled); from T. feicki it differs by its smaller size (SVL to 389 vs. to 448 mm), in the less distinctive neck (HW/NW 1.6 vs. 1.8–2.2), proportionately smaller eyes (ED/HW 0.20 vs. 0.28–0.32), and in the higher number of both spot rows (4 vs. 1) and body spots (43–46 vs. 18–29).
Diagnosis (in Spanish). Una especie de Tropidophis de talla mediana, de hábito estilizado (cuerpo algo comprimido lateralmente), cabeza diferenciada del cuello y hocico relativamente largo, ojos pequeños, un conteo alto en escamas ventrales, coloración de fondo dorsal pálida de amarillo-bronceado a bronceado-marrón con un patrón de sólo cuatro manchas marrones, las manchas al medio del dorso a veces en contacto o fusionadas, y las manchas laterales mucho más pequeñas y tenues; vientre amarillo, y sin patrón ventral ni cefálico.
Tropidophis leonae sp. nov. requiere comparación cercana con la única otra especie en la Hispaniola, T. haetianus (Figs. 3, 4 y 5). Difiere de la parapátrica T. haetianus en (Tab. I): 1) talla más pequeña (SVL hasta 389 vs. hasta 552 mm en T. haetianus); 2), un mayor número de escamas ventrales (216–217 vs. 170–194); 3) el hábito estilizado y algo comprimido lateralmente (vs. robusto y cilíndrico); 4) cabeza más diferenciada del cuello (HW/NW 1.6 vs. 1.3–1.5); 5) hocico más largo (SNL/HL 0.34 vs. 0.30–0.34); 6) ojos proporcionalmente más pequeños (ED/ HW 0.20 vs. 0.21–0.30, ED/HL 0.12 vs. 0.13–0.16, ED/SNL 0.36 vs. 0.41–0.50, ED/IND 0.68– 0.72 vs. 0.79–1.04); 7) distancia interorbital más estrecha (IOD/HW 0.46–0.47 vs. 0.48–0.60, IOD/HL 0.28 vs. 0.30–0.36); 8) conteo de hileras de manchas más bajo (4 vs. 8–10, raramente 6 en T. haetianus); 9) color de manchas (chocolate marrón vs. tonos de verde-olivo jaspeado de negro); 10) hilera lateral de manchas pequeñas y tenues (vs. manchas grandes y bien definidas en T. haetianus; Fig. 3); 11) área dorsal de la cabeza sin patrón, casi uniformemente naranjabronceado (vs. cabeza más oscura que el dorso, y suele poseer figuras oscuras); 12) área lateral de la cabeza casi uniformemente bronceado-marrón, jaspeado de amarillo (vs. con una máscara o barra ocular de marrón-olivo oscura a negruzca contrastando con escamas labiales y garganta muy pálidas); 13) carente de patrón ventral (pecas pequeñas e irregulares amarillo-bronceadas limitadas a los bordes de algunas escamas vs. manchas grandes y muy oscuras; Fig. 4). La otra especie geográficamente cercana a T. leonae sp. nov. es T. bucculentus (de la isla Navaza), de la cual difiere por una menor talla (SVL hasta 389 vs. hasta 596 mm), un hábito estilizado (vs. robusto), un mayor número de escamas ventrales (216–217 vs. 183–186 en T. bucculentus), la cabeza más diferenciada del cuello (HW/NW 1.6 vs. 1.5), escamas dorsales lisas (vs. aquilladas), y en tener tanto un menor número de filas de manchas (4 vs. 6) como de manchas del cuerpo (43–46 vs. 48–54).
Otras especies del Caribe en las que se justifica comparación por producirse solapamiento con Tropidophis leonae sp. nov. en el conteo de escamas ventrales, se descartan de la siguiente manera: T. leonae sp. nov. se distingue de T. wrighti por tener una talla más pequeña (SVL hasta 389 vs. hasta 488 mm en T. wrighti; Dominguez et al., 2005), ojos proporcionalmente más pequeños (ED/HW 0.20 vs. 0.32–0.34), la cabeza más diferenciada del cuello (HW/NW 1.6 vs. 1.8–2.2), y un mayor número de manchas del cuerpo (43–46 vs. 21–37); de T. semicinctus difiere por tener los ojos proporcionalmente más pequeños (ED/HW 0.20 vs. 0.30–0.34), un mayor número de hileras de manchas (4 vs. 2), y más manchas del cuerpo (43–46 vs. 17–26); de T. melanurus difiere en la talla mucho más pequeña (SVL hasta 389 vs. >1000 mm; Díaz & Cádiz, 2020; Rodríguez-Cabrera et al., 2021) y en la condición de las escamas dorsales (lisas vs. aquilladas); de T. feicki difiere en la talla más pequeña (SVL hasta 389 vs. hasta 448 mm), la cabeza más diferenciada del cuello (HW/NW 1.6 vs. 1.8–2.2), ojos proporcionalmente más pequeños (ED/HW 0.20 vs. 0.28–0.32), y tanto en un mayor número de hileras de manchas (4 vs. 1) como de manchas del cuerpo (43–46 vs. 18–29).

Figure 1. Dorsal view of the full body of Tropidophis leonae sp. nov. A) Holotype; B) typical substrate at the habitat floor in the type locality. The matching pattern provides good camouflage.

Figure 2. Tropidophis leonae sp. nov. (holotype) submerged in a water hole in the limestone floor. Note the arrows pointing at some toad (Peltophryne armata) metamorphs and tadpoles. The snake was observed feeding on the tadpoles and also striking at the toadlets.
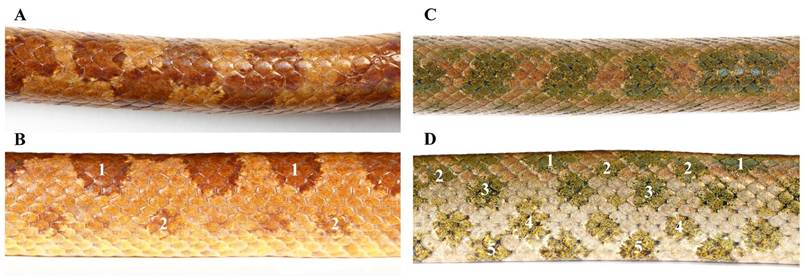
Figure 3. Dorsal and lateral partial views of Tropidophis leonae sp. nov. (A, B, respectively) and T. haetianus (C, D) in life, showing coloration and pattern. Numbers in B and D point out the number of spot rows on one side only.

Figure 4. Ventral views of A) Tropidophis leonae sp. nov. (MNHNSD 23.3951), and B) T. haetianus (MNHNSD 23.3967). Note the lack of pattern in A, whereas large discrete dark spots are present in B.

Figure 5. Lateral view of the head of A) Tropidophis leonae sp. nov. (MNHNSD 23.3951) showing the nearly uniformly colored head, and B) that of T. haetianus (MNHNSD 23.3967) with a distinctive dark cap, and a dark mask or facial horizontal stripe across the eye.

Figure 6. Habitat at the type locality of Tropidophis leonae sp. nov. The blue arrow in the photo at the right points out the water hole where the holotype was found (in Fig. 2).

Figure 7. Some behaviors exhibited by Tropidophis leonae sp. nov. (MNHNSD 23.3951) with an apparent defensive purpose. A) Autohemorrhaging when manipulated; B) dorsoventral body flattening against the substrate after being disturbed when first encountered.
Table I. List of selected characters diagnosing Tropidophis leonae sp. nov. from T. haetianus (data mainly from Hedges 2002, and from samples in Appendix 1)
This paper Hedges 2002
|
Character |
Tropidophis leonae sp. nov. (N = 2) |
Tropidophis haetianus (N = 25) |
Tropidophis haetianus (N = 158) |
|
SVL (maximum) |
389 |
509 |
552 |
|
HW/NW |
1.6 |
1.2–1.5 (21) |
1.3–1.5 (8) |
|
ED/HW |
0.20 |
0.21–0.30 (22) |
0.22–0.25 (8) |
|
ED/HL |
0.12 |
0.13–0.16 (24) |
- |
|
ED/SNL |
0.36 |
0.41–0.50 (24) |
- |
|
ED/IND |
0.68–0.72 |
0.79–1.04 (24) |
- |
|
IOD/HW |
0.46–0.47 |
0.48–0.60 (23) |
- |
|
IOD/HL |
0.28 |
0.30–0.36 |
- |
|
IND/IOD |
0.59–0.63 |
0.45–0.56 (24) |
- |
|
Ventrals |
216–217 |
175–194 (26) |
170–194 |
|
Midbody scale rows |
24–25 |
25–29 (26) |
25–29 [23 or 29] |
|
Body spot rows |
4 |
8–10 (26) |
8–10 [6] |
|
Body spots |
43–46 |
38–61 |
36–61 |
|
Facial stripe/mask |
absent |
present |
present* |
|
Body spot color |
chocolate-brown |
olive-brown to pale olive, with blackish freckling |
dull olive-brown to pale olive; dark olive; dark olive-brown* |
Asterisk indicates that data (unknown sample size) were gathered from Schwartz & Marsh (1960). Numbers in parentheses indicate sample sizes other than column top. Brackets denote rarely recorded values.
Description of the holotype. Size medium (SVL 362 mm), habitus slender, body somewhat laterally compressed, head distinctive from neck (HW/NW ratio 1.6), snout long (SNL/HL 0.34), eyes relatively small (ED/HW 0.20 and ED/HL 0.12), not protruding from head edge in dorsal view; dorsal scales smooth, 217 ventral scales, 24 midbody scale rows, undetermined subcaudal count (mutilated tail). One small interparietal scale, parietal shields in contact with each other; 1/1 preocular, 2/2 postoculars; 7 circumorbitals; frontal scale more than twice the width of one supraocular, 1/1supraoculars, 9/9 supralabials, first supralabial almost as high as the second, 9/9 infralabials.
Coloration in life. Metachrotic: dorsal ground color pale yellowish-tan when submerged in water, or brownish-tan when exposed. The following description is based on the individual while in captivity: four spot rows, dorsalmost blotches milk-chocolate brown, some of which are in contact middorsally, and some fused in parallel (giving the impression of a single transverse band or ovate blotch), irregularly outlined with darker edges and finely bordered (but not entirely encircled) with pale, yellowish cream; a lateral row of one smaller, rather faint spot, and at times a small, isolated and diffuse accessory smudge appears on one side only (never arranged in a row). Head patternless, orange-tan, with tan-brown stippling, its sides without a mask or dark stripe across (horizontally) the eye. Supralabial scales with some yellow and brown flecking, infralabials grayish-yellow with dark brown flecking, which increases and concentrates towards each scale’s anterior edge. Venter pale yellow, posterior edges of ventral scales ivory-yellow, with no pattern other than small tan to tan-brown smudges limited to both the basal edges and to the outer, lateral edges of the ventral scales. Throat ivory-yellow with brown stippling, chin shields brighter yellow, each of the first chin shields with one dark brown spot at their junction with first infralabial scales, central gulars with more brown stippling, and the posterior gulars a much cleaner ivory-yellow continuous to first ventrals.
Coloration in preservative. Dorsum pale yellowish-tan, venter pale ivory-yellow, throat ivorygrayish with brown stippling. Dorsalmost blotches nearly as described in life.
Variation. Size medium (SVL 362–389 mm). The paratype (MNHNSD 23.3951) agrees in most respects with the description of the holotype, differing from it in having 3/3 postorbitals; the dorsal ground coloration in life was tan, but this individual was about to shed at the moment of capture, as it showed a duller hue and wrinkled scales. Days later in alcohol the layer of skin came loose from the body.
Etymology. The epithet honors Dominican biologist and friend Yolanda (a.k.a “Yoli”) M. León, a tireless advocate of conservation efforts in the Dominican Republic and whose support also contributed to this work.
Distribution. The new species is only known from the type locality, in the karst foothills of Loma Las Trincheras, Paso Sena, 4 km N of Pedernales, Pedernales Province.
Natural history notes. The habitat is dry forest on a limestone rocky floor (Fig. 6). More information on the habitat and its associated herpetological community appears in Landestoy et al. (2021). The first individual encountered (MNHNSD 23.3951) was partially exposed from a crevice in a limestone bedrock at 2000 h (Fig. 7-B). The second specimen (holotype, MNHNSD 23.3952) was found at 2200 h submerged in a rainwater filled hole in the bedrock. This hole and nearby bedrock also contained both tadpoles and metamorphs of the Hispaniolan Armored Toad (Peltophryne armata; Fig. 2), which were preyed upon by the snake (see below).
When first encountered, this individual was light yellowish-tan, but then darkened (tan-brown) after capture. Likewise, specimen MNHNSD 23.3951 was yellowish-tan when encountered and then turned tan-brown in captivity. Similar color change has been reported in several Tropidophis species (Hedges et al., 1989; Rehak, 1987).
Foraging and diet. MNHNSD 23.3952 struck at toadlets that were outside (at the edge) of the water hole, but also at tadpoles underwater. In one instance, it captured one tadpole by the side and went into the bottom debris of the water hole, seeking concealment to swallow its prey. The dimensions of the aperture were 32×21 cm, and the hole was 6.5 cm deep (though the water level was about half as deep). On the second night of captivity, the snake consumed three toad metamorphs within one hour while enclosed in a plastic bag that contained a little amount of water along with four metamorphs and four tadpoles.
Defensive behavior. Body flattening against the limestone rock surface: when disturbed, it dorsoventrally flattened itself against the rocky substrate, either to decrease body mass exposed above surface level or, alternatively, to increase adhesion to substrate with ventral scales (Fig. 7-B); also, performs lateral body compression, sidewinding locomotion (fleeing from captor), musk from cloaca (white to clear fluid), coiling into a ball with head concealed, and auto-hemorrhaging (blood expelled from mouth and eyes; Fig. 7-A). The dorsal pattern and coloration appear to camouflage well with the ground substrate, where Acacia seedpods abound (Fig. 1).
DISCUSSION
Based on observations of the diversity, morphological distinctiveness and ecological patterns in the Cuban species of Tropidophis, three ecotype categories – terrestrial, generalist, and semi-arboreal – were proposed by Rodríguez-Cabrera et al. (2016; 2020). As I explain below, several morphological characters of Tropidophis leonae sp. nov. conform with the semi-arboreal ecotype, except for the small eyes (Hedges, 2002). Tropidophis leonae sp. nov. possesses a somewhat laterally compressed body and a relatively thin neck compared with the head, two features generally associated with arboreality (lateral compression: Díaz & Cádiz, 2020; Hedges & Garrido, 1992; Lillywhite & Henderson, 1993; Pizzato et al., 2007; thin neck: Díaz & Cádiz, 2020; Hedges & Garrido, 1992). There is also a high number of ventral scales in arboreal Tropidophis species (Hedges, 2002), with species in the semi-arboreal ecotype possessing ventral scale counts approaching or exceeding 200. A high ventral scale count is also observed in the small, arboreal Hispaniolan Chilabothrus (Landestoy et al., 2021), although a separate study on arboreal boids and pythonids did not detect this same pattern (Pizzato et al., 2007). The high ventral count in T. leonae sp. nov. agrees with the slenderness of its habitus and the more distinctive neck, and this combination of features is not observed in the predominantly terrestrial T. haetianus (Henderson & Powell, 2009). The new species is both smaller and more slender than T. haetianus, and likewise differs in the HW/NW ratio and some head proportions. While its morphology seems to most strongly align with arboreality, the only two available field observations of T. leonae sp. nov., were of individuals on the ground (limestone rocks), although substrate affinities cannot yet be robustly inferred. Nonetheless, given these observations of habitat use, a more laterally compressed body could confer an adaptive advantage for a rockdwelling lifestyle, perhaps through more efficient use of it for foraging or for access to crevices as refuges, as the species has been seen actively hunting in a water-filled hole on the limestone floor.
Other species of snakes that co-occur with T. leonae sp. nov. are Chilabothrus striatus, Ch. ampelophis, Hypshyrinchus ferox, Uromacer frenatus, U. oxyrhynchus, Typhlops sp. and Mitophis pyrites. There are records of T. haetianus around Pedernales near the type locality of Tropidophis leonae sp. nov. (Schwartz & Henderson, 1991), and there is a recently collected specimen (MNHNSD 23.3967) 9 km N of the type locality at a higher elevation (260 m asl) and in a mesic situation (riparian habitat).
Of the 17 Tropidophis species in Cuba, up to four are often found in sympatry (RodríguezSchettino et al., 2013); hence, ecological partitioning among congeners appears more evident there (Hedges & Garrido, 1992; Rodríguez-Cabrera et al., 2016). Hispaniola, by contrast, has been known to harbor only one species (T. haetianus), which is predominantly terrestrial and is often found sympatric with two species of the locally more diverse (four species) boid genus Chilabothrus (personal observation). In Chilabothrus, microhabitat specialization is linked to body size and shape as well as head shape (Chandler & Tolson, 1990; Landestoy et al., 2021; Reynolds et al., 2016; Rodríguez-Robles et al., 1996). Three species of these boas have evolved small sizes and represent ecological specialists on Hispaniola (Henderson et al., 1987; Landestoy et al., 2021; Reynolds et al., 2016; Tolson, 1987), which likely occupy similar niches otherwise used by at least one Tropidophis species in the assemblages found in Cuba, where a single, large generalist species of Chilabothrus occurs (Rodríguez-Cabrera et al., 2016). The habitat and range of Tropidophis leonae sp. nov. is confluent with that of the Hispaniolan Vineboa (Chilabothrus ampelophis), a recently described slender and small-bodied boa (Landestoy et al., 2021), for which the type locality is the same as for T. leonae sp. nov., and both species have been found just meters away from each other, although the former has been observed on bushes and trees (at least two individuals have been seen using rocks as refuges elsewhere, MALT unp. data) and the latter has been observed on the ground (limestone rocks). The ecology of these two poorly known species has not been studied yet, limiting any conclusions.
With Tropidophis leonae sp. nov., a total of four species of herpetofauna have been described from the Barahona Peninsula within the last five years (Landestoy et al., 2021; Landestoy et al., 2022; Landestoy et al., 2018), underscoring the Barahona Peninsula as a herpetological hotspot that remains, unfortunately, quite understudied. The type locality of the Hispaniolan Armored Toad (Peltophryne armata) lies in the vicinity of the type locality of both T. leonae sp. nov. and Chilabothrus ampelophis. For the latter, an allopatric speciation scenario has been suggested with C. fordii (Landestoy et al., 2021), as occurs with other species with distributions limited to the Barahona Peninsula and with closely related species north of this region (Schwartz, 1980; Viñola-López & Almonte, 2022). It is possible that the other recently discovered species have allopatric counterparts from the North Paleo-island or even to the north of the peninsula either within the Sierra de Bahoruco or its northern versant or associated lowlands. The pattern involving this scenario has been known as the Barahona Entrapment (Landestoy et al., 2021; Schwartz, 1978; 1980). Furthermore, is noteworthy that all three species are threatened, and at least one of them, P. armata, has been formally assessed by the IUCN red list under the category of Critically Endangered (IUCN SSC Amphibian Specialist Group, 2022). For the remainder, the same category would also apply since similar threats are shared.
Further specimens and DNA analysis will shed light into the relationships of this new species. To date, phylogenetic relationships of T. leonae sp. nov. cannot be inferred. However, a sister relationship with its neighbor species, T. haetianus, would not be surprising despite the large extent of their phenotypic divergence.
ACKNOWLEDGEMENTS
The author’s
fieldwork in the Pedernales region has been held with the assistance of skilled
local field biologist Yimell Corona. Special gratitude is also owed to
colleagues Gerson Féliz, Robert Ortíz, Francis Ortíz. Our local colleagues
Wilkin Terrero and Starlin Mena also helped with field assistance and
logistics, especially Robert Perez, in whose family property (the type
locality) we often overnighted, allowing us to conduct work without moving
around during curfews imposed by the authorities during the COVID-19 pandemic.
Martha Muñoz and Javier Torres helped improve the initial draft of this
manuscript. Daniel Perez-Gelabert provided information that led us to valuable
specimens used for comparisons. Paola Villaman contributed a specimen from a
class assignment. The family at Hostal Doña Chava makes us always feel part of
their own. We also thank INTEC-Grupo Jaragua for partial support through the
project “Technology and citizen science” funded by the PEER/NAS/USAID Program.
Carlos Suriel and Cristian Marte from the Museo Nacional de Historia Natural
“Prof. Eugenio de Jesús Marcano” provided research facilities and access to
relevant specimens. The Ministerio de Medio Ambiente y Recursos Naturales of
the Dominican Republic granted research permits. Finally, thanks to the
anonymous referees for their valuable comments to improve this manuscript.
REFERENCES
Chandler, C. R. & Tolson, P. J. (1990). Habitat use by a boid snake, Epicrates monensis, and its anoline prey, Anolis cristatellus. Journal of Herpetology, 24(2), 151–157.
Cochran, D. M. (1941). The herpetology of Hispaniola. Bulletin of the Smithsonian Institution United States National Museum.
Cocteau, J. T. & Bibron, G. (1843). Reptiles. In R. Sagra (Ed.) De la, Historia Física, Política y Natural de la Isla de Cuba. Tomo IV. Reptiles y Peces. Segunda parte (pp. 1–142). Arthus Bertrand.
Curcio, F. F., Sales Nunes, P. M., Suzard Argolo, A. J., Skuk, G., & Trefaut Rodrigues, M. (2012). Taxonomy of the South American dwarf boas of the genus Tropidophis Bibron, 1840, with the description of two new species from the Atlantic forest (Serpentes: Tropidophiidae). Herpetological Monographs, 2012(26), 80–121.
Díaz, L. M. & Cádiz, A. (2020). A new species of Tropidophis (Squamata: Tropidophiidae) and molecular phylogeny of the Cuban radiation of the genus. Novitates Caribaea, (16), 1–9.
https://doi.org/10.33800/nc.vi16.222
Domínguez, M., Moreno, L. V., & Sánchez, M. (2005). Tropidophis wrighti (NCN). Size record. Herpetological Review, 36(2), 197.
Hedges, S. B. (2002). Morphological variation and the definition of species in the snake genus Tropidophis (Serpentes, Tropidophiidae). Bulletin of the Natural History Museum [London], Zoology Series, 68(2), 83–90.
Hedges, S. B. (2022). CaribHerp. West Indian amphibians and reptiles. Retrieved August 4, 2022, from http://www.caribherp.org/
Hedges, S. B., Estrada, A. R., & Díaz, L. M. (1999). New snake (Tropidophis) from Western Cuba. Copeia, 1999(2), 376–381.
Hedges, S. B. & Garrido, O. H. (1992). A new species of Tropidophis from Cuba (Serpentes: Tropidophiidae). Copeia, 3, 820–825. https://doi.org/10.2307/1446158
Hedges, S. B. & Garrido, O. H. (1999). A new snake of the genus Tropidophis (Tropidophiidae) from central Cuba. Journal of Herpetology, 33(3), 436–441.
Hedges, S. B. & Garrido, O. H. (2002). A new snake of the genus Tropidophis (Tropidophiidae) from eastern Cuba. Journal of Herpetology, 36(2), 157–161.
Hedges, S. B., Hass, C. A., & Maugel, T. K. (1989). Physiological colour change in snakes. Journal of Herpetology, 23, 450–455.
Hedges, S. B., Powell, R., Henderson R. W., Hanson S., & Murphy J. C. (2019). Definition of the Caribbean Islands biogeographic region, with checklist and recommendations for standardized common names of amphibians and reptiles. Caribbean Herpetology, 67, 1–53. https://doi.org/10.31611/ch.67
Henderson, R. W., Inchaustegui, S., & Landestoy T., M. A. (2021). Tropidophis haetianus.
The IUCN Red List of Threatened Species 2021: e.T75606510A75608024. https://dx.doi. org/10.2305/IUCN.UK.2021-2.RLTS.T75606510A75608024.en
Henderson, R. W., Noeske-Hallin, T. A., Ottenwalder, J. A., & Schwartz, A. (1987). On the diet of the boa Epicrates striatus on Hispaniola, with notes on E. fordi and E. gracilis. Amphibia-Reptilia, 8(3), 251–258.
Henderson, R. W. & Powell, R. (2009). Natural history of West Indian amphibians and reptiles. University Press of Florida.
IUCN SSC Amphibian Specialist Group. (2022). Peltophryne armata. The IUCN Red List of Threatened Species 2022: e.T172817035A172817040. https://dx.doi.org/10.2305/IUCN. UK.2022-1.RLTS.T172817035A172817040.en
Landestoy T., M. A., Reynolds, R. G., & Henderson, R. W. (2021). A small new arboreal species of West Indian boa (Boidae; Chilabothrus) from southern Hispaniola. Breviora, 571, 1–20. https://doi.org/10.3099/MCZ67.1
Landestoy T., M. A., Schools, M., & Hedges, S. B. (2022). A new genus and species of Caribbean forest lizard (Diploglossidae; Celestinae) from southern Hispaniola. Zootaxa, 5219(3), 201–226. https://doi.org/10.11646/zootaxa.5219.3.1
Landestoy T., M. A., Turner, D. B., Marion, A. B., & Hedges, S. B. (2018). A new species of Caribbean toad (Bufonidae, Peltophryne) from southern Hispaniola. Zootaxa, 4403(3), 523–539.
Lillywhite, H. B. & Henderson, R. W. (1993). Behavioral and functional ecology of arboreal snakes. In R. A. Seigel and J. T. Collins, (Eds.) Pp. 1–48. Snakes: ecology and behavior. New York, NY: McGraw Hill Inc.
Pizzatto, L., Almeida-Santos, S. M., & Shine, R. (2007). Life-history adaptations to arboreality in snakes. Ecology, 88, 359–366.
Rehak, I. (1987). Color change in the snake Tropidophis feicki (Reptilia: Squamata: Tropidophiidae). Vestník Ceskoslovenské spolecnosti zoologické, 51, 300–303.
Reynolds, R. G., Collar, D. C., Pasachnik, S. A., Niemiller, M. L., Puente-Rolón, A. R., & Revell, L. J. (2016). Ecological specialization and morphological diversification in Greater Antillean boas. Evolution, 70, 1882–1895.
Rodríguez-Cabrera, T. M., Marrero, R., & Torres, J. (2016). An overview of the past, present, and future of the Cuban Boa, Chilabothrus angulifer (Squamata: Boidae): A top terrestrial predator on an oceanic island. Reptiles & Amphibians, 23(3), 152–168.
Rodríguez-Cabrera, T. M., Saval, E. M., Navarro, R. A., Piggot, J. Q., Rodríguez-González, A. M., & Torres, J. (2021). Giant dwarfs: very large giant tropes Tropidophis melanurus (Squamata: Tropidophiidae), and new maximum size records for the species. Reptiles & Amphibians, 28(3), 404–410.
Rodríguez-Cabrera, T. M. & Torres, J. (2020). New dietary records for three Cuban snakes in the genus Tropidophis (Tropidophiidae), with comments on possible niche partitioning by Cuban tropes. Reptiles & Amphibians, 27(2), 201–208.
Rodríguez-Robles, J. A., Greene, H. W., Powell, R., & Henderson, R. W. (1996). Ecological patterns in Greater Antillean macrostomatan snake assemblages, with comments on bodysize evolution in Epicrates (Boidae). Contributions to West Indian herpetology: a tribute to Albert Schwartz. Contributions to herpetology, 12, 339–357.
Rodríguez-Schettino, L., Mancina, C. A., & Rivalta-González, V. (2013). Reptiles of Cuba:
checklist and geographic distributions. Smithsonian Herpetological Information Service, 144, 1–98.
Schwartz, A. (1975). Variation in the Antillean boid snake Tropidophis haetianus Cope. Journal Herpetology, 9(3), 303–311.
Schwartz, A. (1978). Some aspects of the herpetogeography of the West Indies. Academy of Natural Sciences, Philadelphia, Special Publication, 13, 31–51.
Schwartz, A. (1980). The herpetogeography of Hispaniola, West Indies. Studies of the Fauna of Curaçao and Other Caribbean Islands, 189, 86–127.
Schwartz, A. & Henderson, R. W. (1991). Amphibians and reptiles of the West Indies. Descriptions, distributions, and natural history. University of Florida Press.
Schwartz, A. & Marsh, R. J. (1960). A review of the pardalis-maculatus complex of the boid genus Tropidophis of the West Indies. Bulletin of the Museum of Comparative Zoology, 123(2), 49–84.
Stull, O. G. (1928). A revision of the genus Tropidophis. Occasional Papers of the Museum of Zoology, Univiversity of Michigan, 195, 1–49 + 3 figs.
Tolson, P. J. (1987). Phylogenetics of the boid snake genus Epicrates and Caribbean vicariance theory. Occasional Papers of the Museum of Zoology of the University of Michigan, 715, 1–68.
Uetz, P., Freed, P., & Hošek, J. (2022). The Reptile Database. Available: http://www. reptiledatabase.org, (accessed: august 4, 2022).
Viñola-López, L. W. & Almonte, J. N. (2022). Revision of the fossil land tortoises (Testudines: Testudinidae) from Hispaniola with the description of a new species, Dominican Republic. Novitates Caribaea, (20), 11–29. https://doi.org/10.33800/nc.vi20.302
Citation: Landestoy T., M. A. (2023). A remarkable new snake of the genus Tropidophis (Squamata: Tropidophiidae) from southern Hispaniola. Novitates Caribaea, (21), 1–17. https://doi.org/10.33800/ nc.vi21.323
APPENDIX 1
SPECIMENS EXAMINED FOR MORPHOLOGICAL COMPARISONS
Tropidophis haetianus (n = 26): Dominican Republic: Barahona: MNHNSD 23.3964–3966, Platón, 7 km NNW Paraiso (N18.04512° W71.20436°; 225 m above sea level); Distrito Nacional: MNHNSD 23.3097, Villa Mella, El Tamarindo; MNHNSD 23.3960, Parque Mirador Sur, Santo Domingo (N18.43503° W69.97725°; 48 m asl); Elías Piña: MNHNSD 23.701, Pedro Santana; Espaillat: MNHNSD 23.3954, Villa Cafetalera, Amaceyes (N19.50084° W70.52887°; 668 m asl); Hato Mayor: MNHNSD 23.3956, 1 km WNW Paraíso Caño Hondo (N19.05894° W69.46320°; 34 m asl); Independencia: MNHNSD 23.2893, Jimaní, Laguna El Limón; MNHNSD 23.3953, MNHNSD 23.3968, Rabo de Gato, Puerto Escondido (N18.31341° W71.58337°; 400 m asl); Monseñor Nouel: MNHNSD 23.3955, MNHNSD 23.3957–23.3958, Río Colorado (cerca balneario), Los Cacaos, Hatillo, (N18.94559° W70.21623°; 128 m asl); Pedernales: MNHNSD 23.3959, road to Fondo Paradí, 3.7 km SSW Los Tres Charcos (N17.79690° W71.45577°; 105 m asl), MNHNSD 23.3963, Los Tres Charcos (roadkill, found dead on the main road), MNHNSD 23.3967, La Agüita, 13 km N of Pedernales (N18.15512° W71.74661°; 260 m asl); Puerto Plata: MNHNSD 23.700, Los Mameyes, Loma Isabel de Torres; Samaná: MNHNSD
23.699, Cayo Levantado, MNHNSD 23.769, Sánchez, MNHNSD 23.3195, La Mina, MNHNSD 23.3962, Las Galeras, (N19.28395° W69.19281°; 21 m asl); San Cristóbal: MNHNSD 23.822; San Francisco de Macorís: MNHNSD 23.3194, Loma Quita Espuela, Los Bracitos; Santiago: MNHNSD 23.702, Licey; Valverde: MNHNSD 23.3961, Río Gurabo, near the ranger station (caseta) of Refugio de Vida Silvestre Furnia de Gurabo (N19.49439° W71.17754°; 157 m asl).
