INTRODUCTION
The genus Chelonoidis, is one of the best-known tortoise genera, especially because of the diversity of forms inhabiting the Galapagos Islands and the impact they had on Darwin and his subsequent development of the theory of evolution. Chelonoidis also has three species that live on the mainland, C. carbonarius with a wide distribution from Central America to Northern Argentina, C. denticulatus which is found in the Amazon basin, and C. chilensis distributed in Argentina, Bolivia, and Paraguay (Ernst, 1998; Ernst & Leuteritz, 1999a, 1999b; Vargas Ramírez et al., 2010). Multiple fossil taxa have been described from Central America, South America, and the West Indies, and the oldest fossil remains available of the genus are from the Late Oligocene (de la Fuente et al., 2018). This, along with molecular studies, suggests that the arrival of Testudines to South America from Africa took place between the Late Eocene and the Early Oligocene (Lourenco et al., 2012).
The West Indies had a high diversity of endemic land tortoises during the Quaternary, similar to that found in the Galapagos Islands today. Fossils of Chelonoidis have been found in the multiple island banks in the Bahamas, Turks and Caicos Islands, and the islands Hispaniola, Navassa Island, Cuba, Mona, Sombrero, Anguilla, and Barbados. Hitherto, six species of large and medium-size tortoises have been named: Chelonoidis cubensis (Leidy, 1868) from multiple localities in Cuba, C. sombrerensis (Leidy, 1868) from Sombrero Island, C. monensis (Williams, 1952) from Mona Island, C. alburyorum Franz and Franz, 2009 from Abaco in the Bahamas, with two subspecies in Turks and Caicos C. a. keegani and C. a. sementis (Franz et al., 2020), and C. marcanoi Turvey, 2017 and C. dominicensis Albury et al., 2018 from Hispaniola. All native species of Chelonoidis in the West Indies are extinct today, but radiometric dates of multiple populations of C. alburyorum across the Bahamian archipelago indicate that this species survived until 920–780 BP (Steadman et al., 2020).
Molecular studies using ancient DNA of specimens of C. alburyorum from numerous islands in the Bahamas and Turks and Caicos Islands confirmed the assignation of this species to Chelonoidis. It also suggested that the separation between the ancestor of C. alburyorum and its sister clade conformed by C. chilensis and C. niger took place between the early and the Middle Miocene (Kehlmaier et al., 2021; Kehlmaier et al., 2017). Kehlmaier et al. (2017) further proposed that Chelonoidis arrived in the West Indies either thru Northern South America or Central America. Several authors have suggested that the species from the Greater Antilles share morphological characteristics with continental congeners and not with the taxa from the Bahamas, and Turks and Caicos Islands (Albury et al., 2018; Franz & Franz, 2009; Franz et al., 2020). However, it is not clear yet whether Chelonoidis colonized the region multiple times, or the diversity observed in the West Indies is the result of a single event followed by the radiation of species across islands.
In Hispaniola, fossils of Chelonoidis were first described by Franz and Woods (1983), but it was not until 2017, after the discovery of new isolated elements from the southwest Dominican Republic, that a new species was described, C. marcanoi (Turvey et al., 2017). However, Vlachos (2018) and Albury et al. (2018) considered C. marcanoi a nomen dubium because the holotype of the species, a humerus (Fig. 2), is not a diagnostic element to distinguish among species of the genus. At the same time, Albury et al. (2018) named a new species, C. dominicensis, from a nearly complete specimen comprising the carapace with associated cranial and postcranial elements recovered in an underwater deposit in Oleg’s Bat Cave, in La Altagracia Province, northeastern of the Dominican Republic. Here we revise the taxonomy of Hispaniola extinct turtles utilizing new specimens collected from new and former localities.

Figure 1. Map of Hispaniola with localities containing elements of Chelonoidis included in this paper. Cueva del Papayo (1, type locality of C. marcanoi), Cueva de las Tortugas (2), Cueva Gabot (2), Cueva del Mono (3, type locality of C. gersoni), Oleg’s Bat Cave (4, type locality of C. dominicensis [= C. marcanoi]), Cueva del Pastor (5).
OBJECTIVES
-To revise the taxonomy of Quaternary land tortoises from Hispaniola and describe a new species.
MATERIAL AND METHODS
Abbreviations and terminology. Collection acronyms: AMNH, American Museum of Natural History, New York; CJOL, Colección Johanset Orihuela León, Florida; CLV, Colección Lázaro Viñola, Cuba; MHD, Museo del Hombre Dominicano; MNHNCu, Museo Nacional de Historia Natural de Cuba, Cuba; MNHNSD, Museo Nacional de Historia Natural “Profesor Eugenio de Jesús Marcano”, Santo Domingo, Dominican Republic; NHMUK, Natural History Museum, London; NMB, National Museum of the Bahamas/Antiquities, Monuments and Museums Corporation, Abaco, the Bahamas; UF, Florida Museum of Natural History, University of Florida, Gainesville.
Material examined. C. alburyorum alburyorum: Sawmill Sink, UF 225400 (Holotype, carapace, skull, and postcranial skeleton), NMB.AB50.T4 (shell). C. alburyorum keegani UF 453000 (Holotype, complete plastron), UF 452989 (anterior plastron), UF 452991 (anterior plastral lobe), UF 452990 (partial plastron), UF 452991 (anterior plastral lobe), UF 453010 (left humerus), UF 453011 (right humerus), UF 432468 (left mandible). C. alburyorum sementis UF 432441 (Holotype, left xiphiplastron), UF 432462 (left epiplastron), UF 432455 (epiplastron). C. carbonarius. C. chilensis UF 33621, 33603, 33615, 33609, 33600, 33605. C. cubensis: Cienfuegos Province, Ciego Montero, AMNH 6202 (anterior lobe of the plastron), 6203 (xiphiplastron), 6204 (nuchal); Stancti Spiritus Provnice, Casimba de las Llanadas, AMNH 13078 (three partial humerus), 13079 (three partial humerus); Matanzas Province, Cueva Centella, CLV 2230 (entoplastron), 2233 (scapula), CJOL P281 (entoplastron); Cueva Afan, CLV 2457, 2516 (right humerus), 2563, 2536 (left humerus); Cantera J4 No. 1 CLV 2531 (left humerus), 94 (scapula), 2582 (right dentary), Cantera J4 No. 2, CLV 2606 (left maxilla). C. denticulatus UF 19241, 33685, 33680. C. niger UF 19507, 116066, 141684. Centrochelys sulcata UF 55250, 159339, 153852.
Here we follow the skeletal terminology and measures used by Joyce and Bell (2004), Hulbert (2001), Franz and Franz (2009), and Albury et al. (2018). Franz and Franz (2009) used the term “bird face” referring to the interclavicular sculpture of the entoplastron, which has an anterior more elevated mass and an interclavicular keel in the midline with a pair of lateral fossae. This structure is diagnostic at species level and the term “bird face” has been adopted by researchers describing fossil of Chelonoidis in the Bahamas and Greater Antilles (Albury et al., 2018; Franz et al., 2020; Turvey et al., 2017).
Localities. The specimens described here were collected from five localities in the Dominican. Specimens from two of those localities, Cueva de las Tortugas and Oleg’s Bat Cave, have been reported before and a description of the deposits and associated fauna can be found in Turvey et al. (2017) and Albury et al. (2018), respectively. Two of the new localities, Cueva del Mono and Cueva Gabot, are sinkhole caves localized in the Jaragua National Park, Pedernales Province in the southwest Dominican Republic. Both localities lie in the southeastern portion of the southern paleo-island in Hispaniola (Fig. 1). Jaragua National Park is dominated by a karstic landscape with abundant sinkholes and vertical caves that open in Miocene-Pleistocene marine limestone (Cooke et al., 2018). The fauna and geology of Cueva del Mono have been described elsewhere (Cooke et al., 2018; Steadman et al., 2019) and include Late Pleistocene-Mid Holocene sloths, rodents, a primate, numerous lizards, and a giant eagle. Cueva Gabot is very similar in origin to the former one. The cave-sinkhole has a 12 m fall from the entrance on the surface. Fossils of late Quaternary tortoises, rodents, sloths, lizards, and birds were collected from three excavation pits (60 cm x 60 cm) at the bottom of the cave. The fifth locality, Cueva del Pastor, localized in Paraje Hoyo Claro in the Municipality of Juanillo, La Altagracia Province is a partially flooded cave. Fossil remains of Crocodylus rhombifer, Isolobodon portoricensis, and a tortoise were collected from the wet part of the cave by Juan Almonte, Solanlly Carrero and Philip Lehman, in August of 2021. The associated fauna of mammals from the five caves is very similar and characteristic of other late Pleistocene-Holocene deposits in Hispaniola.
In addition to the localities mentioned above, Franz and Woods (1983), and Turvey et al. (2017) reported remains of Chelonoidis from several caves from Dominican Republic. Although those specimens were referred to C. marcanoi, most of the remains are to incomplete or not diagnostic at species level. Similarly, there are fossils of Chelonoidis from several other localities housed at the MNHNSD but can not be identified beyond genus level.
RESULTS
Systematic paleontology
Order Testudines
Family Testunidae
Genus Chelonoidis
The fossil tortoises described here are assigned to Chelonoidis instead of Hesperotestudo or any other North America taxa because they lack cervical scute, limb and tail armor, and growth annuli. They also possess thin shells, pectoral scute narrow at the midline and wider antero-posteriorly towards the marginal scutes, enlarged entoplastron and pectoral scute, and interior entoplastral sculpture (Franz & Franz, 2009; Vlachos, 2018).
Chelonoidis marcanoi Turvey, Almonte, Hansford, Scofield, Brocca, et Chapman, 2017 (Figs. 1, 2, 3, 4)
Chelonoidis marcanoi Turvey et al., 2017: 1, 4, 5; Albury et al., 2018: 2, 3, 4, 21, 22; Franz et al., 2020: 2; Vlachos, 2018: 46, 82.
Chelonoidis dominicensis Albury et al., 2018: 7; Morgan et al., 2018: 3, 43; Franz et al., 2020: 2, 7, 9; Kehlmaier et al., 2021: 2, 4, 7.
Emended diagnosis. Medium-size tortoise of the Greater Antilles Chelonoidis group (C. cubensis, C. gersoni sp. nov.) based on the presence of thin shell, prominent bony ridges in costal sulci, and strong epiplastral shelf that separates gular from the internal floor of the lobe. It differs from other species in the group based on the presence of an oval entoplastron (subtriangular in C. cubensis) with a prominent two-part brow on the upper part of the entoplastron and a very thin keel separating a prominent pair of fossae (one low brow with a wide and low keel separating a shallow pair of fossae in C. gersoni sp. nov.). Humerus with shallow muscle scar for M. latissimus dorsi and medial process significantly above the humeral head.
Diagnosis enmendada (in Spanish): Tortuga de tamaño mediano del grupo Chelonoidis de las Antillas Mayores (C. cubensis, C. gersoni sp. nov.) basado en la presencia de caparazón delgado, crestas óseas prominentes en los surcos costales y fuerte plataforma en el epiplastron que separa el proceso gular del piso interno del lóbulo anterior. Se diferencia de otras especies del grupo por la presencia de un entoplastron ovalado (subtriangular en C. cubensis) con una ceja prominente en dos partes en la parte superior del entoplastrón y una quilla muy delgada que separa un par de fosas prominentes (una frente baja con una quilla ancha y baja que separa un par de fosas poco profundas en C. gersoni sp. nov.). Húmero con cicatriz muscular del M. latissimus dorsi poco profunda y proceso medio significativamente por encima de la cabeza del humero.
Description
Detail description of the morphology of the shell and the skull of this species can be found in Albury et al. (2018).
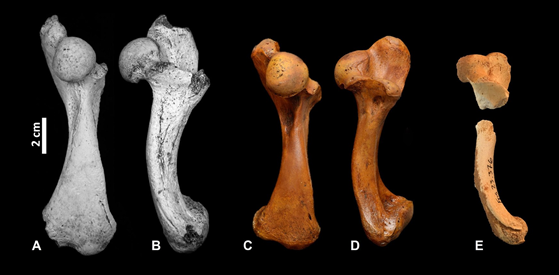
Figure 2. Left humerus of the type specimens of Chelonoidis. C. marcanoi (A, B, NHMUK PV R 36954), C. dominicensis (C, D, MHD 1000), and C. gersoni sp. nov. (E, MNHNSD FOS 23.001).
Skull. The premaxilla is triangular, and it has a very shallow cup-like fossa on the internal side (Fig. 3). The premaxilla and the maxilla are separated by a reduced transverse ridge. The maxilla has three straight and parallel cutting edges, the tomial edge (more lingual), the middle triturating edge, and the lingual edges like in C. alburyorum whereas they are concave in C. cubensis. The tomial and middle triturating edges are reduced in C. marcanoi but more prominent in C. cubensis and C. alburyorum. The jugal process of the maxilla is small in C. marcanoi while very prominent in C. alburyorum and absent in C. cubensis. The contact suture between the maxilla and the jugal is not exposed laterally like in C. cubensis. The posterior margin of the exoccipital, opisthotic, and squamosal is slightly concave similar to C. alburyorum. The anterior edge of the condyle ends well behind the anterior edge of the tympanic cavity.
Dentary. The dentary and right articular of C. marcanoi resemble that of other Chelonoidis in being elongated, narrow, and relatively shallow while that of C. alburyorum is more robust (Fig. 3). It is characterized by the presence of a frontal large “tooth” or beak surrounded by a lateral depression on each side in the labial edge of C. alburyorum and C. chilensis, while the lateral depressions are reduced or absent in other species. Most of the labial cutting edge, including the two depressions, possess well-defined indentation but the lingual cutting edge is free of indentation. The lingual and labial cutting edges are straight, parallel to each other like in C. alburyorum but in C. cubensis they are slightly concave. Both cutting edges are separated by a wide and deep channel. In lateral view, the section of the lingual edge posterior to the depression is nearly straight and at the same as the more internal edge. The posterior half of the ventral margin of the dentary is ventrally directed. In internal view, a prominent Meckelian sulcus separates the upper and wider section of the dentary from the lower and thinner section like in C. alburyorum whereas in C. cubensis this sulcus is very shallow. The small inferior alveolar foramen is in the posterior section of the dentary near the shelf formed by the Meckelian sulcus, and the articular also contributes to it. The specimen MHD–1000, on which this description is based, is part of the type series of C. dominicensis but was not included in the original description.

Figure 3. Comparative plate of cranial and mandibular elements of Chelonoidis. C. alburyorum (A-F, UF 225400), C. marcanoi (type specimen of C. dominicensis MHD 1000, G-L), and C. cubensis (M-R, CLV 2606, 2582).
Shell and postcranial skeleton. The shell of this medium size tortoise is thin like in other West Indies species of the genus and possesses a caudal bump that begins at suprapygal (similar in C. gersoni sp. nov.) (Fig. 4). The cervical dent of the nuchal is reduced. The gulars are sub-rectangular and strongly project anteriorly and are separated from the floor of the anterior lobe by a marked epiplastral shelf (as in C. cubensis). The junction of the gular branching is well separated from the entoplastron (as in C. cubensis and C. gersoni sp. nov.). The entoplastron is oval and wider than long as in C. gersoni sp. nov. and C. alburyorum. Humeral scutes are longer than femoral scutes. The xiphiplastral notch is marked as in C. gersoni sp. nov. and C. cubensis, whereas the anal notch is particularly deep and wide in the males of C. marcanoi. The first dorsal vertebrae have a short and wide centrum, lack a central keel, and have relatively reduced prezygapophyses. In the scapula, the angle between acromion and blade is approximately 120° as in C. cubensis and possesses a pelvis with a nearly circular ischiopubic fenestra.
Morphological variation of the plastron. The morphology of the anterior lobe of the plastron is variable among specimens in the sample available. In smaller specimens that probably belong to juveniles the gular is less projected and more rounded than in larger specimens (Fig. 5). This variation was also observed among individuals of different sizes in C. cubensis from Cuba by Williams (1950). However, the larger entoplastra can be segregated into two different classes, one with gular lobes projected laterally and strongly bifurcated with a V notch at the midline, and others with poor or no bifurcated gular lobes which have nearly strait lateral margins (Fig. 4). Similar variation is also present in other extinct tortoises like C. gringorum from the Miocene of Argentina and has been interpreted to be linked with sexual dimorphism (Oriozabala et al., 2017). This morphology seems to vary within and between populations of C. dominicensis across its range (Figs. 1, 5). Individual variation among the sample includes the presence of raised ridges in the ventral side of the plastron MNHNSD FOS 23.107–108 and ventrally inflated gular lobes in specimen MNHNSD FOS 23.214.
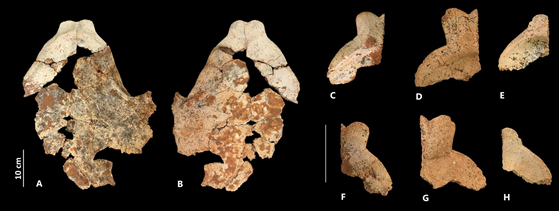
Figure 4. Plastral elements of Chelonoidis marconoi. Partial plastron (MNHNSD FOS 23.107-08) and epiplastrons (Cueva Gabot: C, F, MNHNSD FOS 23.103; D, G, MNHNSD FOS 23.214; E, H, MNHNSD FOS 23.207) of C. marcanoi from Cueva Gabot in the Pedernales Province in internal (A, C, D, E) and ventral (B, F, G, H) view.
Holotype. Right humerus (NHMUK PV R 36954) collected on May 12 of 2007 in Cueva del Papayo, Pedernales Province, Dominican Republic.
Material examined. Pedernales Province: Cueva Gabot, partial plastron missing xiphiplastron and portions of hypoplastron, hyoplastron, and entoplastron (MNHNSD FOS 23.107–108), articulated left epiplastron and entoplastron (MNHNSD FOS 23.303 three left epiplastron (MNHNSD FOS 23.103, 23.207, 23.214), a right epiplastron (MNHNSD FOS WN), and a nuchal (MNHNSD FOS 23.215); Cueva de las Tortugas, two left epiplastron (MNHNSD FOS WN, 23.252), and a right epiplastron (MNHNSD FOS 23.300). La Altagracia Province: Oleg’s Bat Cave, shell and associated skeleton (Holotype of C. dominicensis, MHD–1000); Cueva del Pastor, left entoplastron and associated entoplastron (MNHNSD FOS 23.404).
Distribution. Fossils of this species have been collected from several localities in the southwestern Dominican Republic, Pedernales Province, specifically Cueva del Papayo, Cueva de la Tortuga and Cueva Gabot, and the northeastern Hispaniola, in La Altagracia Province in Oleg’s Bat Cave and Cueva del Pastor (Albury et al., 2018).
Remarks. Chelonoidis marcanoi was described by Turvey et al. (2017) from a nearly complete right humerus (holotype) and several other shells and postcranial elements collected from five caves in the Pedernales Province, in the southwest Dominican Republic. In addition, Turvey et al. (2017) also included the type series of other elements previously reported by Franz and Woods (1983) from a cave near Bayaguana in the Monte Plata Province. Shortly after the publication of C. marcanoi, Albury et al. (2018) and Vlachos (2018) considered the species a nomen dubium because the elements used to erect it are morphologically similar and poorly diagnostic among species of Chelonoidis. Furthermore, Vlachos (2018) noticed that two specimens (MNHNSD FOS 23.1056, MNHNSD FOS 23.1060) identified as epiplastrons by Turvey et al. (2017) correspond to other parts of the shell. We agree with Vlachos (2018) that those species were misidentified, and they correspond to a nuchal (MNHNSD FOS 23.1056) and a peripheral (MNHNSD FOS 23.1060). This also explains the “absence” of the internal ornamentation and gular projections reported for the epiplastron of C. marcanoi by Turvey et al. (2017), which are otherwise characters present in Chelonoidis and other tortoises (Franks & Franks, 2009).
Based on a nearly complete skeleton recovered from Oleg’s Bat Cave, a flooded cave in the La Altagracia Province in the east of the Dominican Republic, Albury et al. (2018) described a new species of tortoise, C. dominicensis. Although the humerus of C. dominicensis cannot be separated from that of C. marcanoi, Albury et al. (2018) suggested that the island of Hispaniola may have sustained more than one species based on biogeographical grounds. The type specimen of C. marcanoi and C. dominicensis come from localities in the southern and northern paleo-islands respectively. Both paleo-islands are currently separated by a large depression, the Neiba Valley, which was periodically inundated until it reached its current configuration in the Late Pleistocene (Graham, 2003; Iturralde-Vinent, 2006; Iturralde & MacPhee, 1999). Although the paleo–island has shared flora and fauna, there are numerous instances where each one hosts closely related species of plants (Judd, 2007), amphibians, reptiles (Landestoy et al., 2018; Schwartz, 1980), and birds (Sly et al., 2011).
Additional fieldwork in several dry caves in the Pedernales Province and a flooded cave in the La Altagracia province led to the discovery of numerous diagnostic fossils of Chelonoidis. An associated entoplastron and epiplastron recovered from Cueva del Pastor in La Altagracia is very similar to the holotype of C. dominicensis, whereas new specimens from Pedernales suggest that two species of Chelonoidis inhabited the region in the Late Pleistocene-Early Holocene. The shell and limb bones of one of them are indistinguishable from C. marcanoi and C. dominicensis whereas several other fossils including an associated skeleton belong to a new form described below. The humerus and shell of the new species fall outside of the range of variation observed in the two previously named species. However, we did fail to find a combination character that could be used to separate the two already described species of Chelonoidis other than small differences in the gular notch. Therefore, we consider C. dominicensis Albury et al., 2018 a junior synonym of C. marcanoi Turvey et al., 2017. Although C. dominicensis was based on a more complete material (associated shell, skull, and limb bones), the name C. marcanoi takes priority over the former.
As mentioned above, within the sample of specimens collected in caves from Pedernales some specimens have strongly projected gulars with marked notch separating them and while others have smaller gulars and less prominent notch (Fig. 4). However, there also seems to be a geographic variation of this morphology. The type specimen of C. dominicensis, a male from the eastern side of the island (Northern Paleo-island), has strongly projected gulars but lacks the midline notch, which contrasts with the gulars of possible males from Pedernales (Southeast Paleo-Island), (Fig. 1). A second specimen from the east of the island has a small notch in the midline of the gular. Pedernales and the eastern side of the island have very different ecosystems. Pedernales is dryer and dominated by xerophytic forest whereas the eastern side of the island is more mesic. The presence of strongly divergent gulars, which would have been covered by a thick sheet of keratin while the animal was alive, may be related to higher pressure for the competition of the limited resources. On the other hand, there are genetic and body size differences between populations of mammals from the North and Southeast paleo–islands that have been interpreted as differences at the subspecies level. This may also be the case for the populations of C. marcanoi here understudy, but increased sample size is necessary to test this hypothesis.
Chelonoidis gersoni sp. nov.
(Figs. 1, 2, 5, and 6)
Zoobank Nomenclatural Act: A8C87F3D–F526–4553–8ABA–D4E35A97F4B7 (June, 2022)
Diagnosis. Medium-size tortoise (greater length of the plastron: 28.12 cm) of the Greater Antilles Chelonoidis group (C. cubensis, C. marcanoi) based on the presence of thin shell, prominent bony ridges in costal sulci, and strong epiplastral shelf that separates gular from the internal floor of the lobe. It can be distinguished from other species in the group based on the presence of pronounced gular projection with triangular lobes (sub rectangular in C. cubensis and C. marcanoi); oval entoplastron (sub triangular in C. cubenisis) with low brow, wide and low middle keel and shallow lateral fossae. Humerus with shallow muscle scar for M. latissimus dorsi (prominent in C. cubensis) and medial process at the same level of the humeral head (medial process higher than humeral head in other species of the genus).
Diagnosis (in Spanish). Tortuga de tamaño mediano del grupo Chelonoidis de las Antillas Mayores (C. cubensis, C. marcanoi) basado en la presencia de caparazón delgado, crestas óseas prominentes en los surcos costales y fuerte plataforma en el epiplastron que separa el proceso gular del piso interno del lóbulo anterior. Se puede distinguir de otras especies del grupo por la presencia de una proyección gular pronunciada con lóbulos triangulares (subrectangulares en C. cubensis y C. marcanoi); entoplastrón ovalado (subtriangular en C. cubenisis) con frente baja, quilla ancha y media baja y fosas laterales poco profundas. Húmero con cicatriz muscular poco profunda para M. latissimus dorsi (prominente en C. cubensis) y proceso medio del humero al mismo nivel de la cabeza del humero (proceso medio significativamente más elevado en otras especies del género).
Description
Plastron. The plastron has a total length of 28.12 cm and is very thin at the midline of the hyoplastron (2.38–2.74 mm). Although it has multiple fractures and part of some scutes are missing, most of the diagnostic elements are well preserved (Fig. 5). The epiplastron is well preserved although it lacks part of the right humeral scute. The gular lobes are strongly projected towards with and strong epiplastral notch like in C. cubensis and C. marcanoi, but the lateral margins of the lobes are tilted medially giving the gular projection a triangular shape, a condition unique among species of Chelonoidis. The apices of the gular lobes are slightly separated by a weak notch between them, and the midline of the gular branching is longer than two lateral branches. At the same time, gulars are widely separated from the entoplastron. In internal view, the epiplastral shelf that separates the gular from the posterior lobes’ excavation is very strong like in C. cubensis and C. marcanoi, while in C. alburyorum it is almost absent. The humeral scute is shorter than in C. marcanoi.
The entoplastron is oval, wider than long, very shallow anteriorly (5.32 mm) and posteriorly (3.81 mm), slightly concave, and does not contact the humero-pectoral groove. The bird face structure of the entoplastron has been used recently to distinguish among species of Chelonoidis by Franz and Franz (2009) and Albury et al. (2018). The sculpture in C. gersoni sp. nov. is unique among species of Chelonoidis, it includes a low individual brow in the anterior section of the entoplastron, a very shallow and wide beak that ends in a free spine before reaching the posterior section of the entoplastron, and very shallow fossae. It markedly differs from C. cubensis and C. marcanoi which bears elevated brow and beak with deep fossae.
The hyoplastron is poorly preserved, missing most of its lateral section, but it is very thin near the midline. Like in other members of Chelonoidis the pectoral scute is narrower at the midline and expands towards the more lateral section of the scute (Zacarias et al., 2013). The humeral scute (67.4 cm) is nearly as long as the femoral scute (62.14 cm) like in C. alburyorum, and it is a character commonly used to distinguish some species of Chelonoidis (Albury et al., 2018). On the other hand, the hypoplastron is very well preserved, despite the presence of numerous internal fractures the pieces remain in anatomical position and can be distinguished clearly. The plastron thickens from the abdominal to the femoral scute. In lateral view, the contact between the femoral and the inguinal scute is very narrow. The xiphiplastron is very well preserved, missing only part of the anal projections on both sides. Although the condition of the anal projection is possible to observe that the specimen had a strong xiphiplastral notch like in C. cubensis and C. marcanoi while it is very reduced or absent in C. alburyorum and C. monensis. The femora-anal groove is nearly transversal in C. gersoni sp. nov. like in C. alburyorum and C. cubensis while oblique in C. monensis and C. marcanoi. The anal scutes are very short at the midline (9.52 cm) but become longer towards the lateral margin of the plastron, in part because of the combination of the nearly transversal femora-anal groove and the strong wedge anal groove. The anal scutes are very thin and gracile in general, while C. cubensis and C. marcanoi have more robust and thicker anal scutes.
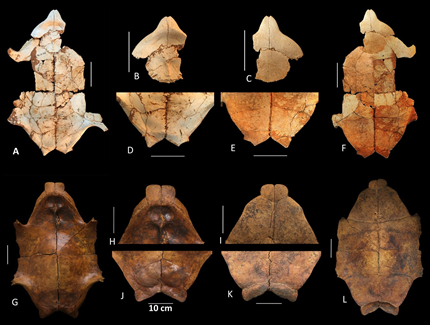
Figure 5. Internal and ventral view of the plastron of Chelonoidis species from Hispaniola. Chelonoidis gersoni sp. nov. (A-F, MHD 1000) and C. marcanoi (G-L, MNHNSD FOS 23.001, holotype). Complete plastron (A, F, G, L), close up of anterior (B, C, H, I) and posterior lobe (D, E, J, K).
Carapace. Only a small portion of the posterior section of the carapace is preserved and includes the pygal, right marginals XI to IV, left marginals XI to VII, and a costal of indeterminate position (Fig. 6). The pygal is domed and in association with marginal XI and X, it seems that form part to be pygal complex bump described known from C. marcanoi. The costal bears the sulci with elevated bony ridges that characterize most species of Chelonoidis from the West Indies except in C. monensis. The marginal XI has the shape of a right trapezoid while the remaining ones are sub-rectangular, in general, all the marginals are thin, particularly those that occupy a more lateral position.

Figure 6. Shell element of C. gersoni sp. nov. (MNHNSD FOS 23.001, holotype). Pygial, right and left peripheral XI, X, IX, VIII, VII, right peripheral VI (A-C), and costal (D).
Humerus. The diaphysis of the humerus is slender and slightly curved like that of C. marcanoi (Fig. 2). The distal end of the M. latissimus dorsi scar is present on the type specimen and suggests that this muscle scar was relatively shallow like C. marcanoi, C. monensis, and C. alburyorum while much deeper in C. cubensis. Differ from all other West Indies forms in the genus hitherto described in that the humeral head and the medial process are at the same whereas the medial process rises significantly above the head in the other species throughout ontogeny.
Types. Holotype. Partial shell (MNHNSD FOS 23.001) of a female that includes the plastron (most of the epiplastron, entoplastron, hypoplastron, xiphiplastron, and midsection of the hyoplastron), pygal, right marginals from XI to IV, left marginals from XI to VII, and a costal of indeterminate position, proximal epiphysis and distal half of right humerus. The specimen was collected by Juan Almonte on August 17 of 2015 in the dry cave Cueva del Mono, Pedernales Province, Dominican Republic. Paratypes, entoplastron (MNHNSD FOS 23.025) from Cueva de Gabot and entoplastron (MNHNSD FOS 23.029) from Cueva de las Tortugas, Pedernales Province, Dominican Republic.
Etymology. We dedicate this species to Gerson Feliz, field biologist of the Grupo Jaragua who has contributed to the study and conservation of the natural resources of Pedernales for over 20 years.
Distribution. Remains of C. gersoni sp. nov. have been collected in the southwestern Dominican Republic, Pedernales Province, in the following caves, Cueva del Mono, Cueva Gabot, and Cueva de la Tortuga.
Remarks. The shape of the gular on the epiplastron and the design of the bird face sculpture on the entoplastron distinguish C. gersoni sp. nov. from any other member of the genus. The diagnostic characters of C. gersoni fall outside the ontogenetic and sexual variation range of C. marcanoi (see Morphological variation of the plastron above) and that of other living and extinct species of Chelonoidis (Barros et al., 2012; Franz et al., 2020; Franz & Franz, 2009; Oriozabala et al., 2017;). It also shares a suite of other characters with species from the West Indies, especially C. cubensis and C. marcanoi, and separates them from C. alburyorum. Hitherto, remains of the species have only been recovered from three caves in the southern paleo-island in Hispaniola. The associated fauna includes another species of tortoise, C. marcanoi, other reptiles like Anolis sp., Leiocephalus sp., Celestus sp., indeterminate snakes; several mammals including Acratocnus ye, Neocnus comes, Plagiodontia araeum, Isolobodon portoricensis, Plagiodontia aedium, P. ipnaeum, Brotomys voratus, Nesophontes hypomicrus, N. paramicrus, N. zamicrus, Solenodon paradoxus, a primate, and the birds Tyto glaucops, Caracara sp., and Falco sp. (Cooke et al., 2018; Turvey et al., 2017; Viñola-Lopez et al., 2022).
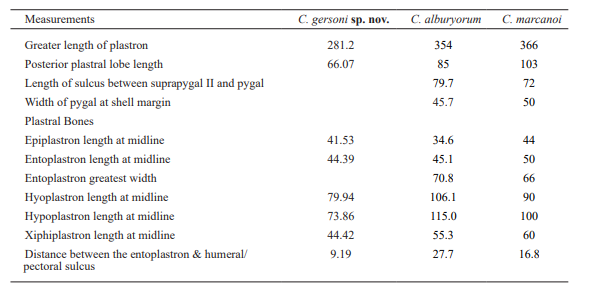
Table I. Shell measurements in mm of Chelonoidis gersoni (MNHNSD FOS 23.001, holotype), C. alburyorum (UF 225400) and C. marcanoi (MHD 1000). Measurements of the last two species were obtained from Albury et al. (2018)
DISCUSSION
Hispaniola is one of the few islands known to have sustained more than one taxon of tortoise. In the Galapagos, Santa Cruz and Isabela also support multiple species of tortoises. There are some cases in those islands where distantly related taxa occupying the same island, suggesting more than one colonization event (Poulakakis et al., 2015). Usually, the taxa found on the same island have an allopatric distribution, with populations separated by altitude, ecological barriers such as humidity content, or living on different sides of the volcanoes (Fritts, 1983; Poulakakis et al., 2015). In the Dominican Republic, fossils of C. marcanoi have been found from La Altagracia Province in the northeastern of the island to Pedernales Province in the southwestern. On the other hand, C. gersoni sp. nov. has been collected only in the Pedernales Province. Remains belonging to both species have been found in Cueva Gabot and Cueva de las Tortugas suggesting that these specimens may have been sympatric. Nonetheless, without a better temporal resolution of deposits where the specimens come from, it is not possible to determine whether the two species overlapped or not.
As Albury et al. (2018) suggested, the presence of two species of Chelonoidis in Hispaniola is not unexpected. The island was bisected longitudinally in two major paleo-islands during the Middle-Late Miocene (Graham, 2003), and it did not reach its current configuration until the late Pleistocene (Maurrasse et al., 1980) The southern paleo-island, composed of Barahona (Dominican Republic) and Tiburon (Haiti) peninsulas, is separated from the northern paleo–island by the Hispaniolan Rift Valley. Although the fauna from each paleo-island may share a common ancestor, this vicariant event often conditioned the evolution of distinctive and endemic biotas in each region. Examples of this are known from plants (Judd, 2007), mammals (Cooke et al., 2011; Turvey et al., 2016), birds (Sly et al., 2011), amphibians, reptiles (Geneva et al., 2015; Parham et al., 2013; Schwartz, 1980), and other groups (Albury et al., 2018; Turvey et al., 2017). On the other hand, the distribution of species from different paleo-islands can overlap in the zone of contact between paleo-islands, as was probably the case of C. marcanoi and C. gersoni in the province of Pedernales. This pattern is also observed in the modern distribution of the terrapins Trachemys decorata and T. stejnegeri, and the rock iguanas Cyclura cornuta and C. ricordii (Parham et al., 2013; Ramer, 2004).
The relationship among species of Chelonoidis in the West Indies is poorly understood, in part because of the lack of comprehensive phylogenetic study and the poor preservation of the fossils recovered from most dry caves. Several of the species described in the region seem to have undergone a high level of specialization, evolving a series of unique characters. This led Williams (1952) to the description of the subgenus Monachelis where he included the fossil tortoise from Mona Island characterized by a very elongated and narrow first dorsal vertebra. On the other hand, Franz and Franz (2009) notices that the skull of C. alburyorum did not have a completely ossified postotic chamber, and proposed that it could have been a hearing adaptation of the Bahamian species. Similarly, C. cubensis, C. marcanoi, and C. gersoni sp. nov. had very distinctive but prominent gular projections, that in extant species are often used during combat (Leuteritz & Gantz, 2013; McRae et al., 1981; Pritchard, 2013). This contrasts with the very reduced gular of C. alburyorum. Furthermore, the Bahamas species also differ from C. cubensis, C. marcanoi, and C. gersoni sp. nov. in that it lacks the strong epiplastral shelf found in the Greater Antilles species and has a more robust dentary. Some of these characters were used by Franz and Franz (2009) and Albury et al. (2018) to argue that Chelonoidis colonized the northern part of the West Indies multiple times independently.
Species of Chelonoidis from the Bahamas and the Greater Antilles had very thin shells compared with other tortoises (Albury et al., 2018; Franz & Franz, 2009; Williams, 1950), which has been interpreted as an adaptation to insular conditions and the lack of predator (Turvey et al., 2017). Nonetheless, C. chilensis, a continental species closely related to C. alburyorum and the Galapagos clade, also have a thin shell. This may suggest that this character evolved independently multiple times under different conditions, or the continental ancestor of the West Indies tortoises had already thin shells before reaching the islands. However, the evolution toward giantism in Chelonoidis is not unique to insular species (Cadena et al., 2015; Zacarias et al., 2013), and not all West Indies tortoises were giant either. C. cubensis, C. sobrerensis and an undescribed species from the Bahamas are the only ones that may fit this description (Auffenberg, 1967; Franz & Franz, 2009; Williams, 1950), with a plastron that probably extended over a meter in length, whereas the Hispaniola species C. marcanoi (greatest length of plastron: 366 mm) and C. gersoni sp. nov. (281.2 mm) were significantly smaller (Albury et al., 2018).
Apart from C. alburyorum, which has become a study model in the region (Franz et al., 2020; Hastings et al., 2014; Kehlmaier et al., 2021; Kehlmaier et al., 2017; Steadman et al., 2020), little is known about the ecology and timing of the extinction of other tortoises in the West Indies (Turvey et al., 2017). Extant members of the genus Chelonoidis occupy a wide range of habitats including savannas, xerophytic and open ecosystems, and wet tropical and subtropical forests (Ernst, 1998; Ernst & Leuteritz, 1999a; 1999b; Fritts, 1983; Manzano et al., 2009; Vargas Ramírez et al., 2010; Wang et al., 2011;). In the Galapagos, Fritts (1983) found a correlation between dryness and the carapace size of tortoises from different islands. Tortoises from more xeric habitats had smaller body sizes while the ones inhabiting more mesic regions grew larger. This correlation was interpreted by Fritts (1983) as a response to ecological pressures like shade and food availability. Similarly, C. gersoni sp. nov. the smallest species of Chelonoidis in Hispaniola has only been found until the moment in various dry areas in the southwestern Dominican Republic. Meanwhile, C. marcanoi is known from both, the xeric and mesic habitats. On the other hand, C. cubensis, a very large tortoise from Cuba, and its remains are often found associated with fauna from open environments (Arredondo, 2007; Orihuela et al., 2020; Viñola-Lopez et al., 2018; Williams, 1950). The gracile and serrated dentaries of C. marcanoi and C. cubensis (Fig. 2) further suggest that the two species potentially ate soft vegetation and fruits, in contrast with the more robust and deeper dentary of C. alburyorum that may have been used to consume harder vegetation. However, all this species with a high diversity of morphologies and ecologies became extinct in the late Pleistocene-Holocene.
In a similar manner in that Chelonoidis from the Galapagos islands are an example of island diversification and radiation, Chelonoidis in the West Indies has become a suitable example for the study of extinction. No other group of reptiles in the West Indies experienced such level of species losses in the late Quaternary and is only comparable with the extinction of some clades of mammals like the Caribbean sloths and primates. While radiocarbon dates and the association of specimens from archaeological contexts in the Bahamas Archipelago suggest that Chelonoidis survived for approximately one to three centuries after the arrival of humans between AD ~700–1000 (Franz et al., 2020; Steadman et al., 2020). The timing and causes of extinction for other species in the region are unknown so far, and the only other species with a radiometric date available is C. marcanoi (Kehlmaier et al., 2021), suggesting that it was still present on Hispaniola around 7810 ± 30 years before the present.
ACKNOWLEDGEMENTS
We want to extend our gratitude to all those who helped to collect the fossils study here as part of the field work supported by the Museo Nacional de Historia Natural “Profesor Eugenio de Jesus Marcano”. Thanks to Jason R. Bourque and William Suarez Duque for the thoughtful discussions on the taxonomy of Hispaniola fossil tortoises. Also, want to thank Laureano Gonzalez Ruiz, Juliana Sterli, and Carolina Oriozabala for sharing images of Chelonoidis gringorum. This manuscript was improved thanks to the input of Carmi M. Thompson, the editor Carlos Suriel and two anonymous reviewers.
REFERENCES
Albury, N. A., Franz, R., Rimoli, R., Lehman, P., & Rosenberger, A. L. (2018). Fossil land tortoises (Testudines: Testudinidae) from the Dominican Republic, West Indies, with a description of a new species. American Museum Novitates, 2018(3904), 1–28.
Arredondo, C. (2007). Paleofauna, paleoambiente y subsistencia alimentaria de humanos tempranos en el noroeste de cuba central. Anthropos 2007. 1 Congreso Iberoamericano de Antropología. (5–9 de marzo de 2007) La Habana, Cuba. CD-ROM: ISBN 959-282-043-0. 679–704.
Auffenberg, W. (1967). Notes on West Indian tortoises. Herpetologica, 23, 34–44.
Barros, M. S., Silva, A. G., & Ferreira Junior, P. D. (2012). Morphological variations and sexual dimorphism in Chelonoidis carbonaria (Spix, 1824) and Chelonoidis denticulata (Linnaeus, 1766) (Testudinidae). Brazilian Journal of Biology, 72, 153–161.
Cadena, E. A., Anaya, F., & Croft, D. A. (2015). Giant fossil tortoise and freshwater chelid turtle remains from the middle Miocene, Quebrada Honda, Bolivia: Evidence for lower paleoelevations for the southern Altiplano. Journal of South American Earth Sciences, 64, 190–198.
Cooke, S. B., Mychajliw, A., Almonte, J. N., Feliz, G., McAfee, R. K., Turvey, S., & Lovett, S. (2018). A Late Pleistocene to Holocene Faunal Community in Pedernales Province, Dominican Republic. Submitted to the 78th Annual Meeting of the Society of Vertebrate Paleontology, Albuquerque, NM.
Cooke, S. B., Rosenberger, A. L., & Turvey, S. (2011). An extinct monkey from Haiti and the origins of the Greater Antillean primates. Proceedings of the National Academy of Sciences, 108(7), 2699–2704.
De la Fuente, M. S., Zacarías, G. G., & Vlachos, E. (2018). A review of the fossil record of South American turtles of the clade Testudinoidea. Bulletin of the Peabody Museum of Natural History, 59(2), 269–286.
Ernst, C. H. (1998). Geochelone chilensis. Catalogue of American Amphibians and Reptiles, 668, 1–4.
Ernst, C. H., & Leuteritz, T. E. J. (1999a). Geochelone carbonaria. Catalogue of American Amphibians and Reptiles, 690, 1–7.
Ernst, C. H., & Leuteritz, T. E. J. (1999b). Geochelone denticulata. Catalogue of American Amphibians and Reptiles, 691, 1–6.
Franz, R., Albury, N. A., & Steadman, D. W. (2020). Extinct tortoises from the Turks and Caicos Islands. Bulletin of the Florida Museum of Natural History, 58(1), 1–38.
Franz, R., & Franz, S. E. (2009). A new fossil land tortoise in the genus Chelonoidis (Testudines: Testudinidae) from the northern Bahamas, with an osteological assessment of other neotropical tortoises. Bulletin of the Florida Museum of Natural History, 49, 1–44.
Franz, R., & Woods, C. A. (1983). A fossil tortoise from Hispaniola. Journal of Herpetology, 17, 79–81.
Fritts, T. H. (1983). Morphometrics of Galapagos tortoises: evolutionary implications. In R. I. Bowman, M. Berson, and A. E. Leviton, (Eds.) Patterns of evolution in Galapagos organisms (107–122). AAAS Pacific Division.
Geneva, A. J., Hilton, J., Noll, S., & Glor, R. E. (2015). Multilocus phylogenetic analyses of Hispaniolan and Bahamian trunk anoles (distichus species group). Molecular phylogenetics and evolution, 87, 105–117.
Graham, A. (2003). Geohistory models and Cenozoic paleoenvironments of the Caribbean region. Systematic Botany, 28, 378–386.
Hastings, A. K., Krigbaum, J., Steadman, D. W., & Albury, N. A. (2014). Domination by reptiles in a terrestrial food web of the Bahamas prior to human occupation. Journal of Herpetology, 48(3), 380–388.
Hulbert Jr., R. C. (Ed.). 2001. The fossil vertebrates of Florida. Gainesville: University Press of Florida.
Iturralde-Vinent, M. A. (2006). Meso-Cenozoic Caribbean paleogeography: implications for the historical biogeography of the region. International Geology Review, 48, 791–827.
Iturralde-Vinent, M. A., & MacPhee, R. D .E. (1999). Paleo-geography of the Caribbean region: Implications for Cenozoic biogeography. American Museum of Natural History Bulletin, 238, 1–95.
Joyce, W. G., & Bell, C. J. (2004). A review of the comparative morphology of extant testudinoid turtles (Reptilia: Testudines). Asiatic Herpetological Research, 10, 53–109.
Judd, W. S. (2007). Revision of Miconia sect. Chaenopleura (Miconieae, Melastomataceae) in the Greater Antilles. Systematic Botany Monographs, 81, 1–235.
Kehlmaier, C., Albury, N. A., Steadman, D. W., Graciá, E., Franz, R., & Fritz, U. (2021). Ancient mitogenomics elucidates diversity of extinct West Indian tortoises. Scientific reports, 11(1), 1–9.
Kehlmaier, C., Barlow, A., Hastings, A. K., Vamberger, M., Paijmans, J. L. A., Steadman, D. W., Albury, N. A., Franz, R., Hofreiter, M., & Fritz, U. (2017) Tropical ancient DNA reveals relationships of the extinct Bahamian giant tortoise Chelonoidis alburyorum. Proceedings of the Royal Society B, 284, 20162235. https://doi.org/10.1098/rspb.2016.2235.
Landestoy T., M. A., Turner, D. B., Marion, A. B., & Hedges, S. B. (2018). A new species of Caribbean toad (Bufonidae, Peltophryne) from southern Hispaniola. Zootaxa, 4403, 523–539.
Leidy, J. (1868). Notice of some vertebrate remains from the West Indian Islands. Proceedings of the Academy of Natural Sciences of Philadelphia, 178–180.
Leuteritz, T. E., & Gantz, D. T. (2013). Sexual dimorphism in radiated tortoises (Astrochelys radiata). Chelonian Research Monographs, 6, 105–112.
Lourenço, J. M., Claude, J., Galtier, N., & Chiari, Y. (2012). Dating cryptodiran nodes: origin and diversification of the turtle superfamily Testudinoidea. Molecular Phylogenetics and Evolution, 62(1), 496–507.
Manzano, A. S., Noriega, J. I., & Joyce, W. G. (2009). The tropical tortoise Chelonoidis denticulata (Testudines: Testudinidae) from the late Pleistocene of Argentina and its paleoclimatological implications. Journal of Paleontology, 83(6), 975–980.
Maurrasse, F., Pierre-Louis, R., & Rigaud, J. G. (1980). Cenozoic facies distribution in the southern peninsula of Haiti and the Barahona Peninsula, Dominican Republic, and its relations concerning tectonic evolution of the La Selle-Baoruco block. Caribbean Geology, Collected Contributions, 9, 1–24.
McRae, W. A., Landers, J. L., & Cleveland, G. D. (1981). Sexual dimorphism in the gopher tortoise (Gopherus polyphemus). Herpetologica, 46–52.
Orihuela, J., Viñola-Lopez, L. W., Vázquez, O. J., Mychajliw, A. M., de Lara, O. H., Lorenzo, L., & Soto-Centeno, J. A. (2020). Assessing the role of humans in Greater Antillean land vertebrate extinctions: New insights from Cuba. Quaternary Science Reviews, 249, 106597.
Oriozabala, C., Sterli, J., & Ruiz, L. G. (2017). Morphology of the mid-sized tortoises (Testudines: Testudinidae) from the Middle Miocene of Northwestern Chubut (Argentina). Ameghiniana, 55(1), 30–55.
Parham, J. F., Papenfuss, T. J., Van Dijk, P. P., Wilson, B. S., Marte, C., Schettino, L. R., & Simison, W. B. (2013). Genetic introgression and hybridization in Antillean freshwater turtles (Trachemys) revealed by coalescent analyses of mitochondrial and cloned nuclear markers. Molecular Phylogenetics and Evolution, 67(1), 176–187.
Poulakakis, N., Edwards, D. L., Chiari, Y., Garrick, R. C., Russello, M. A., Benavides, E., WatkinsColwell, G. J., Glaberman, S., Tapia, W., Gibbs, J. P., & Cayot, L. J. (2015). Description of a new Galápagos giant tortoise species (Chelonoidis; Testudines: Testudinidae) from Cerro Fatal on Santa Cruz island. PLoS One, 10(10), e0138779.
Pritchard, P. C. H. (2013). Madagascar: Island Continent of Tortoises Great and Small. Chelonian Research Monographs, 6, 17–24.
Ramer, J. (2004). A survey of Ricord’s iguanas (Cyclura ricordii) and Rhinoceros iguanas (Cyclura cornuta cornuta) in Isla Cabritos National Park, Dominican Republic 2003: A preliminary report. Iguana, 11, 88–95.
Schwartz, A. (1980). The herpetogeography of Hispaniola West Indies. Studies on the fauna of Curacao and other Caribbean Islands, 61(1), 86–127.
Sly, N. D., Townsend, A. K., Rimmer, C. C., Townsend, J. M., Latta, S. C., & Lovette, I. J. (2011). Ancient islands and modern invasions: disparate phylogeographic histories among Hispaniola’s endemic birds. Molecular Ecology, 20(23), 5012–5024.
Steadman, D. W., Albury, N. A., Carlson, L. A., Franz, R., LeFebvre, M. J., Kakuk, B., & Keegan, W. F. (2020). The paleoecology and extinction of endemic tortoises in the Bahamian Archipelago. The Holocene, 30(3), 420–427.
Steadman, D. W., Almonte Milan, J. N., & Mychajliw, A. M. (2019). An extinct eagle (Aves: Accipitridae) from the Quaternary of Hispaniola. Journal of Raptor Research, 53(3), 319–333.
Turvey, S. T., Almonte, J., Hansford, J., Scofield, R. P., Brocca, J. L., & Chapman, S. D. (2017). A new species of extinct late Quaternary giant tortoise from Hispaniola. Zootaxa, 4277(1), 1–16.
Turvey, S. T., Peters, S., Brace, S., Young, R. P., Crumpton, N., Hansford, J., Nuñez-Miño, J. M., King, G., Tsalikidis, K., Ottenwalder, J. A., Timpson, A., Funk, S.M., Brocca, J.L., Thomas, M. G., & Barnes, I. (2016). Independent evolutionary histories in allopatric populations of a threatened Caribbean land mammal. Diversity and Distributions, 22, 589–602. https://doi. org/10.1111/ddi.12420.
Vargas Ramírez, M., Maran, J., & Fritz, U. (2010). Red-and yellow-footed tortoises, Chelonoidis carbonaria and C. denticulata (Reptilia: Testudines: Testudinidae), in South American savannahs and forests: do their phylogeographies reflect distinct habitats? Organisms Diversity & Evolution, 10(2), 161–172.
Viñola-Lopez, L. W., Garrido, O. H., & Bermudez, A. (2018). Notes on Mesocapromys sanfelipensis (Rodentia: Capromyidae) from Cuba. Zootaxa, 4410(1), 164–176.
Viñola-Lopez, L. W., Bloch, J. I., Almonte, J. N., & LeFebvre, M. (2022). The biogeography and timing of extinction of endemic rodent on Hispaniola during the Holocene. Quaternary Science Reviews. [Manuscript submitted for publication].
Vlachos, E. (2018). A review of the fossil record of North American turtles of the clade PanTestudinoidea. Bulletin of the Peabody Museum of Natural History, 59(1), 3–95.
Wang, E., Donatti, C. I., Ferreira, V. L., Raizer, J., & Himmelstein, J. (2011). Food habits and notes on the biology of Chelonoidis carbonaria (Spix, 1824) (Testudinidae, Chelonia) in the southern Pantanal, Brazil. South American Journal of Herpetology, 6(1), 11–19.
Williams, E. E. (1950). Testudo cubensis and the evolution of western hemisphere tortoises. Bulletin of the American Museum of Natural History, 95(1), 1–36.
Williams, E. E. (1952). A new fossil tortoise from Mona Island, West-Indies, and a tentative arrangement of the tortoises of the world. Bulletin of the American Museum of Natural History, 99(9), 545–560.
Zacarias, G. G., de la Fuente, M. S., Fernández, M. S., & Zurita, A. E. (2013). Nueva especie de tortuga terrestre gigante del género Chelonoidis Fitzinger, 1835 (Cryptodira: Testudinidae), del Miembro inferior de la Formación Toropí/Yupoí (Pleistoceno tardío/Lujanense), Bella Vista, Corrientes, Argentina. Ameghiniana, 50(3), 298–318.
