INTRODUCTION
Species distributions in natural systems are strongly modulated by climate, which ultimately affects both the ecology and physiology of organisms. This is particularly evident in ectothermic animals. Janzen (1967) published one of the most prominent papers in ecology that connected climatic variation across latitude and elevation, physiological adaptation and species distribution in a synthetic theory commonly referred as “Janzen’s hypothesis” (Ghalambor et al., 2006; Muñoz & Bodensteiner, 2019). One of the main predictions of this hypothesis is that due to the decrease in mean annual temperature with elevation, the seasonal temperature overlap is lower in the tropics than in temperate regions. Hence, mountain passes in the tropics may represent more effective physiological barriers to dispersal than the topographical component of change in altitude (Ghalambor et al., 2006). Therefore, the low overlap in temperature regimes between low and high elevations in the tropics should select for organisms with relatively narrow thermal tolerances. Janzen’s hypothesis also predicts that species develop physiological adaptations mirroring the range of ecological variation present in their surrounding area with populations living in high altitude evolving narrow tolerance for colder temperatures while low altitude populations developing narrow tolerance for warmer temperatures. Janzen’s hypothesis has been widely adopted and some studies have provided at least partial evidence at both local and global scale supporting his predictions and assumptions in both terrestrial and aquatic ectothermic organisms. For example, Pintanel et al. (2019) found that frog species occurring in open habitats, such as in valleys and lowland environments in general, had higher tolerance to high temperatures (CTmax) than species restricted to forest habitats, showing small climatic overlap across an elevation gradient. Moreover, Polato et al. (2018) provided strong evidence in support of Janzen’s hypothesis showing that tropical stream insects had noticeably narrower thermal tolerances and a lower dispersal ability than temperate species, which result in higher tropical speciation rates.
However, despite of general support of the theory, several components have never been thoroughly tested and critically evaluated across multiple taxa, potentially questioning the generality of Janzen’s theory (Ghalambor et al., 2006). In addition, under Janzen’s hypothesis is unclear whether the predictions refer to individual thermal niches or species thermal niches, which in fact are determined by different factors (Hua, 2016). In fact, some studies have not found support for Janzen’s theory: in amphibians (Valdivieso & Tamsitt, 1974) and in Anolis lizards from Hispaniola (Muñoz & Bodensteiner, 2019) factors such as daily variation in temperature and behavioral mechanisms might cause deviations from Janzen’s predictions. Furthermore, Navas et al. (2013) demonstrated that the effect of different microclimates within a specific biome is more relevant for species distributions than just the elevation at which certain species of amphibians may be found. McCain (2009) provided additional evidence for the effects of thermoregulation, daily temperature variability, and other climate variables such as precipitation as potential variables that could explain distribution ranges across multiple groups of vertebrates, including mammals, birds, reptiles and amphibians. In other words, Janzen’s theory may have to be amended by including more complexity.
Several key features related to the geographic distribution of Limia make these fishes an excellent system to explore how temperature fluctuations associated to elevational gradients might be linked to dispersal. Limia fishes are one of the most dominant groups in freshwater ecosystems in the Caribbean with at least 19 endemic species on Hispaniola and one endemic species each occurring in Cuba, Jamaica, and Grand Cayman (Burgess & Franz, 1989; Rodríguez, 1997; Hamilton, 2001; Rodriguez et al., 2020). These freshwater fishes occur in a wide distribution range occupying diverse aquatic habitats on these islands (Weaver et al., 2016a).
Even though thermal regimes of Greater Antillean streams are relatively stable, geological differences among islands lead to some climate heterogeneity that can generate environmental barriers. Hispaniola, for example, has several mountain ranges of more than 2000 meters in altitude. For instance, Pico Duarte in the Dominican Republic, the highest peak in the Caribbean, reaches 3098 meters. Previous studies have shown that high elevation specialists (mainly amphibians and reptiles) have evolved on Hispaniola as a consequence of this climate heterogeneity (Wollenberg et al., 2013; Muñoz et al., 2014). Mountains reaching around 2000 meters can be also found in eastern Cuba (Pico Turquino) and Jamaica (Blue Mountains) with significant levels of biodiversity associated.
Although the altitudinal distribution of freshwater fish species in general is known to be considerably more constrained than in terrestrial species by several factors including for example productivity, physicochemical characteristics of the water and others (Jaramillo-Villa et al., 2010; Graham et al., 2014; Carvajal-Quintero et al., 2015), differences in altitudinal distribution in species of the genus Limia can be observed in natural habitats. In the present study, we tested some predictions of the Janzen’s hypothesis at the local scale through the analysis of the individual thermal niche breadth in several populations of livebearing fishes of the genus Limia and its relationship with their altitudinal distribution in some islands of the Greater Antilles in the Caribbean.
According to theory, we hypothesize that populations of species distributed in lowland habitats have evolved to resist higher extreme temperatures, which may be a factor limiting their dispersal into higher elevations. Conversely, populations occurring at higher elevations in mountain streams should have evolved to cope with lower temperatures, which reduce dispersal abilities into warmer habitats. Specifically, we predict that low elevation populations will be more tolerant to higher temperatures than mid and high elevation populations showing higher critical thermal maximum (CTmax) and critical thermal minimum (CTmin). In contrast, high elevation populations are expected to be more tolerant to lower temperatures showing lower CTmax and CTmin values. We also predict the thermal breadth (the range of temperatures they can tolerate) to be smaller for higher altitude fishes as result of little variability in CTmax.
OBJECTIVES
- Test some predictions of the Janzen’s hypothesis at local scales through the analysis of the individual thermal niche breadth in populations of livebearing fishes of the genus Limia and its relationship with their altitudinal distribution in some islands of the Greater Antilles.
MATERIALS AND METHODS
The care and use of experimental animals complied with the University of Oklahoma animal welfare laws, guidelines and policies as approved by Animal Welfare Assurance on file with the Office of Laboratory Animal Welfare under the assurance number A3240-01. Experiments were performed under the approved IACUC protocol R17-011 and specimens were collected in the field as part of surveys of the native livebearing fishes of the Greater Antilles (protocol R18-005). No fishes were euthanized nor surgical procedures were performed.
Study area and species. In this study we analyzed populations of some species such as L. perugiae, L. vittata, L. yaguajali and L. sulphurophila that were reported to live in low elevation, warm environments including saline coastal lagoons. We also included in the analysis other species such as L. zonata and L. melanogaster that were obtained from low to intermediate elevations and often associated to relatively cool springs. Finally, one population of L. dominicensis and another of L. versicolor collected in mountain streams at relatively high elevations were analyzed too (Table I).
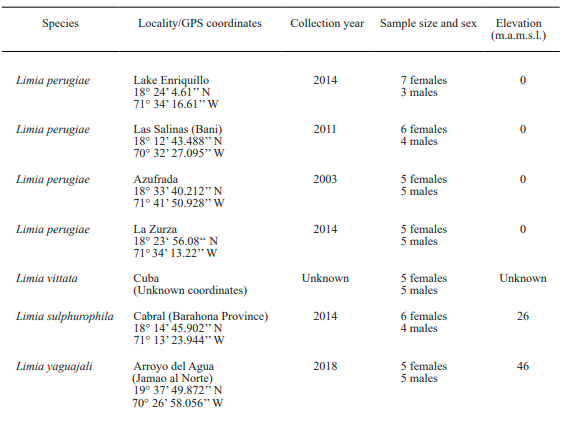
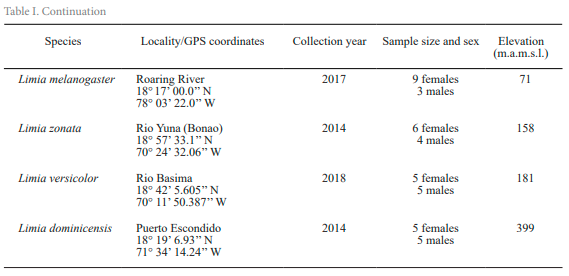
We compared thermal breadth for the eight populations of the Limia species abovementioned that naturally occur in Cuba, Hispaniola, and Jamaica (Fig. 1). For a more fine-grained picture, we analyzed three additional populations of L. perugiae, the most widely distributed species of Limia on Hispaniola in order to test for local adaptation to environmental variation in temperature. All populations analyzed but L. vittata came from wild caught stocks that were transported into the United States and then kept in common garden conditions at the University of Oklahoma for variable periods of time (Table I). L. vittata specimens were obtained from aquarium stocks and have been kept in common garden conditions at a greenhouse in the Aquatic Research Facility at the University of Oklahoma for more than 10 years.
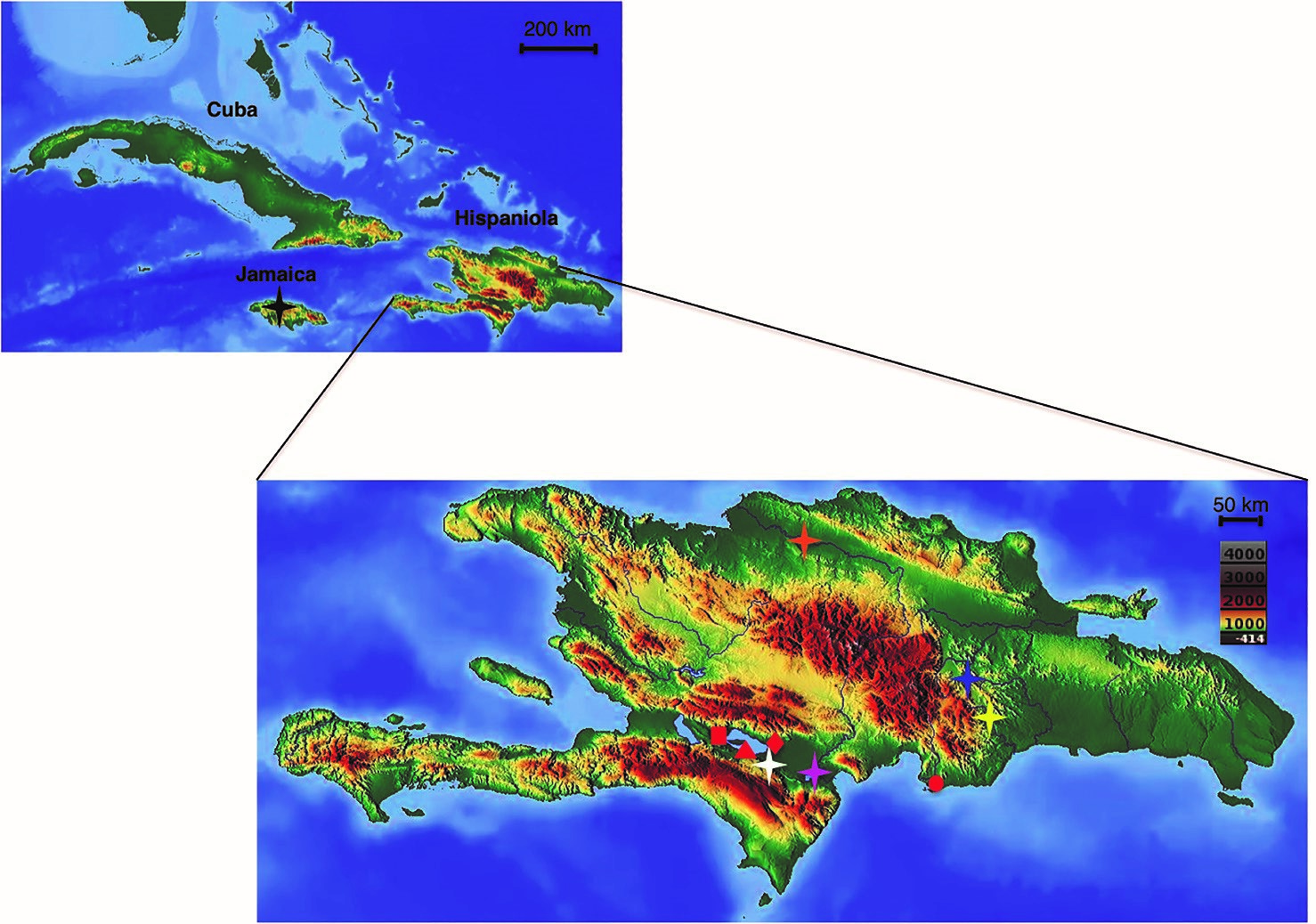
Figure 1. Geographic distribution of the populations of Limia (with known origins) analyzed in this study. Red rectangle: L. perugiae (Azufrada); Red triangle: L. perugiae (Lake Enriquillo); Red diamond: L. perugiae (La Zurza); Red circle: L. perugiae (Las Salinas); Orange star: L. yaguajali; Blue star: L. zonata; Black star: L. melanogaster; White star: L. dominicensis; Yellow star: L. versicolor; Pink star: L. sulphurophila.
Laboratory methods. We used the critical thermal method (Cowles & Bogert, 1944) to describe variation in temperature tolerance in 113 adult fish of eight Limia species representing a total of 11 different populations (Fig. 1). Prior to testing all fishes were acclimated to laboratory conditions with temperatures ranging between 25 °C - 27 °C for 45 days. This was the most common temperature range for the species at their origins. We tested 10 reproductively mature adult fish per population (Table I).
Fishes were individually tested under a constant increase (to determine critical thermal maximum, CTmax) or decrease (to determine critical thermal minimum, CTmin) in temperature until reaching an appropriate endpoint. The endpoint we used to determine CTmax was the pre-death thermal point at which signals of sudden onset of muscular spasms appeared (Lutterschmidt & Hutchison, 1997; Beitinger et al., 2000). In the case of the CTmin the endpoint used was the absence of motion of the pectoral fins in which the fish did not start to move again even when the experimenter disturbed the fish (Fischer & Schlupp, 2009). Using this data, temperature tolerance was calculated as the arithmetic means of high and low temperatures (CTmax or CTmin) at which the endpoint was reached by individuals in the sample (Lowe & Vance, 1955). We also calculated the thermal breadth as CTmax minus CTmin for each individual. The three measures, although connected, reflect different physiological properties of the species.
Before the actual experiment fishes were not fed for 24 hours. Each fish was individually tested in a spherical 2-liter glass container. After a 10-minutes acclimation period before each trial, the container was heated using a concave heating plate at a constant rate of 1 °C/min while temperature was constantly monitored with a thermometer in order to record CTmax. Each trial was immediately stopped, and the final temperature was measured once the fish showed symptoms of sudden muscular spasms, which were characterized by disorganized and high frequency muscular movements. All fish were weighed after each trial, placed in individual tanks, and allowed to rest for at least 72 hours until application of the other extreme temperature to the same individual. Temperature exposure (CTmax or CTmin) that a fish experienced first was randomized to avoid an order effect. This was also evaluated statistically.
A similar procedure was followed to test the fish’s tolerance to cold temperatures or CTmin. In this case, we placed the fish in a similar 2-liter glass container and after a 10-minute acclimation period, we continuously added cold water of 3 4C - 4 5C to the system for a rate of temperature change of 1 °C/min. CTmin was measured at the point in which fish showed total absence of movement. No mortality was associated with the trials and after the experiments all fishes were returned to their respective stock tanks.
Climate index overlap. Monthly maximum and minimum temperatures for the 2010-2019 period were extracted from WorldClim database for the following localities included in Table I: La Zurza, Cabral, Arroyo del Agua, Roaring River, Río Yuna, Río Básima and Puerto Escondido. We calculated the average monthly temperature for each site during the last 10 years in order to measure pairwise thermal overlap between each focal site and the other localities using Janzen’s (1967) equation:
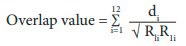
where di is the thermal overlap between the focal site and each other site for the ith month or the amount (in Celsius degrees) of one thermal regime that is included within the other, R1i is the difference between the monthly mean maximum and minimum for the focal site and R2i is the corresponding value for each other site of the study. As temperature overlap increases the overlap value increases up to a value of 12 which is the point where thermal regimes between two sites share identical monthly maximum and minimum temperatures throughout the year.
Phylogenetic signal. A common caveat of studies like this is that any pattern found might not necessarily reflect adaptations but be due to species relatedness. To test for this, we used a previously-inferred phylogeny based on three mitochondrial (12S, ND2, Cytb) and two nuclear (MYH6, Rh) genes and conducted a test of phylogenetic signal to assess whether correlations in temperature tolerance among species may be due to their shared evolutionary history or to other factors (Gingras et al., 2013; Kamilar & Cooper, 2013; Gilbert et al., 2018; Arnaudo et al., 2019). For this analysis we used Pagel’s lambda (λ), (Pagel, 1994) as a quantitative measure of this relationship. The Pagel’s λ has been shown to be a very robust indicator of a correlation between ecological and evolutionary processes even for incompletely resolved phylogenies (Molina-Venegas & Rodriguez, 2017; Leiva et al., 2019). We based this analysis on the phylogeny published by Weaver et al. (2016b), which includes most of the species used in this study.
Data analysis. For data analysis of thermal tolerance, we used one-way ANOVA’s, after confirmation of homogeneity of variances by Levene’s tests and normality by Shapiro and Wilk’s tests. We used three different ANOVAs to compare the mean CTmax, CTmin and temperature ranges (breadth) among eight different populations of Limia. Scheffe’s post hoc tests were used to make comparisons between groups to distinguish populations that differed from others in extreme thermal tolerance or temperature ranges. The lack of order effect was statistically confirmed (p >0.05) using t-test analyses to compare CTmax and CTmin means of fish that were tested CTmax then CTmin versus fish that were tested CTmin then CTmax.
All statistical analyses were performed in SPSS 23. We performed independent ANOVA analyses because we considered CTmax and CTmin as ecologically and evolutionary independent variables with potentially different adaptive benefits. Similar approaches that consider the effects of these variables (and also acclimation temperature) as independent have been used in other studies examining thermal tolerances in ectothermic animals (Spotila, 1972; Layne & Claussen, 1982). To further explore a potential role for local adaptation in thermal tolerance within a widely distributed species, we also compared the same variables through separate ANOVA analyses in four populations of L. perugiae.
In order to calculate climate index overlap we used the R package raster (Hijmans, 2020) to extract minimum and maximum monthly temperatures values to our sampled locations. Finally, analysis of phylogenetic signal was computed using the R package (R Core Team, 2013) phytools (Revell, 2012).
RESULTS
Analysis of phylogenetic signal. The analysis of phylogenetic signal determined whether more similar values in CTmin, CTmax and thermal range were associated with more closely related species more often than expected by chance. None of the analyses found a significant effect of phylogeny in explaining thermal breadth among the Limia species studied: CTmin (λ = 6.257973e-05, p = 1.000), CTmax (λ = 0.4056982, p = 0.78255460) and thermal range (λ =1.16242, p = 0.362986).
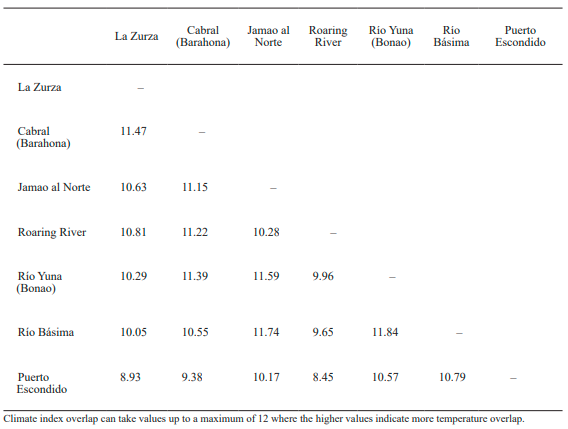
Analysis of climate index overlap. Overall, thermal overlap along altitudinal gradients among the study locations was relatively high but there was still some variation across pair of sites (Table II). Climate index overlap decreased as the differences in elevations were more conspicuous.
Particularly, Puerto Escondido (the highest collecting site included in the analysis) showed the lowest climate overlap with all other sites, which indicates that there are some differences in habitat temperatures.
Table II. Pairwise values of climate index overlap across the seven collecting sites showing different altitudinal gradients
Inter-specific analysis of thermal tolerance. The overall range of temperature tolerance for the eight species included in the analysis was 12°C (CTmin) to 41.2 °C (CTmax), which may be considered as a broad range for tropical fishes when considering the overall climatic stability present in the tropics in terms of temperature fluctuations. A one-way ANOVA was conducted to compare the effect of varying distribution according to elevation on the temperature tolerance under CTmin and CTmax conditions. There were significant differences in thermal limits for both CTmin (One-way ANOVA, F [7, 72] = 41.977, p <0.001) and CTmax (One-way ANOVA, F [7, 72] = 14.878, p <0.001) among species after testing 80 individuals. The highest temperature tolerance was recorded for L. sulphurophila with an average CTmax of 40.9 °C. This species also showed the lowest tolerance to low temperatures with an average CTmin of 16.7 °C, which might suggest this species could be adapted to live in warmer habitats. Post hoc analysis also showed that L. sulphurophila differed significantly from all other species in both CTmin and CTmax (Scheffe, p <0.05) except for L. zonata in CTmin (Scheffe, p = 0.137), (Fig. 2).
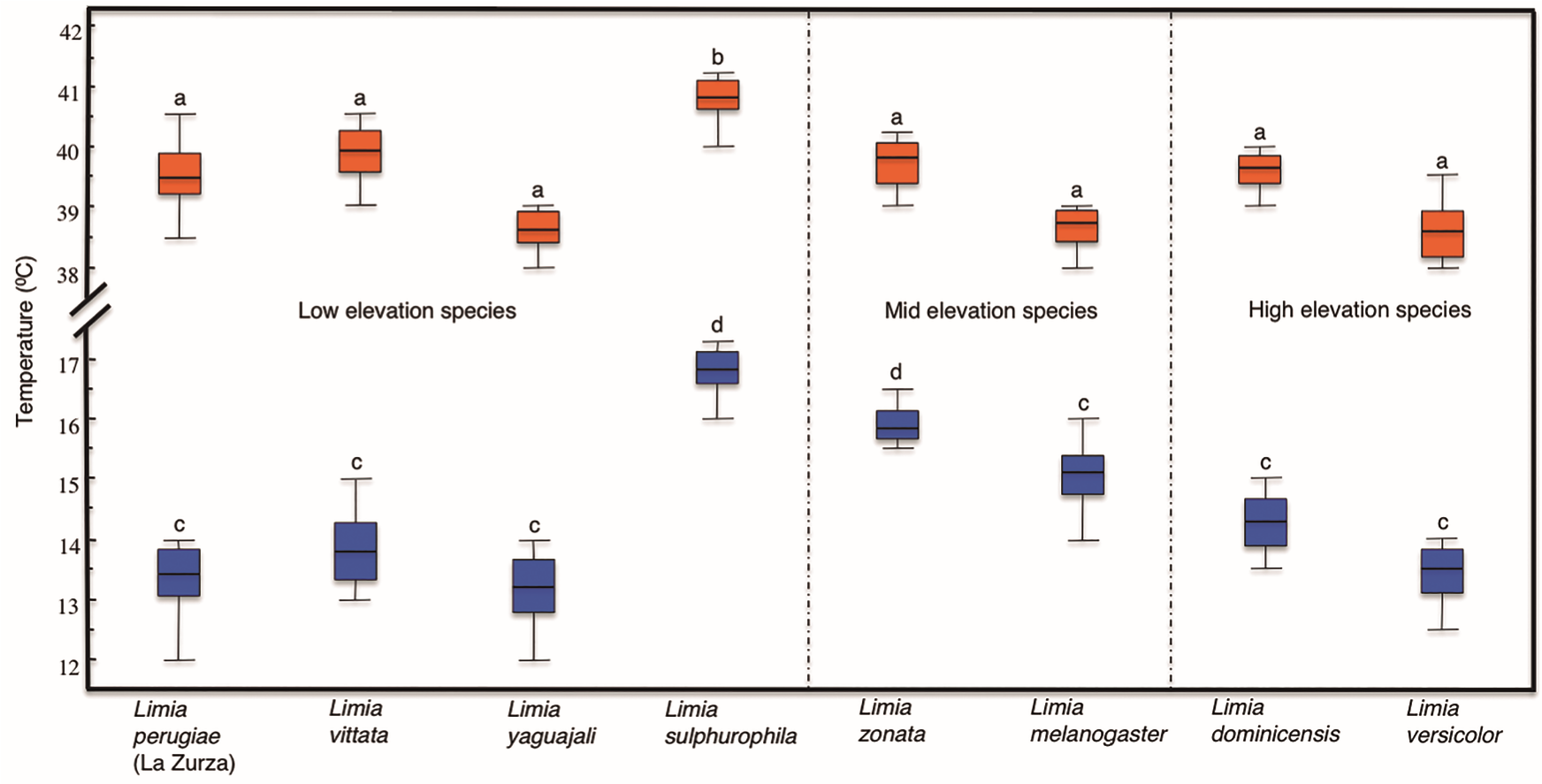
Figure 2. Thermal tolerances of the eight species of Limia included in this study. Species are grouped according to their elevation distribution in three groups: low elevation species (left), mid elevation species (center) and high elevation species (right). Each box plot represents the median, interquartile ranges, maximum and minimum values of either CTmin or CTmax for each species. CTmin is shown by blue box plots and CTmax by orange box plots.
Another ANOVA was used to compare temperature ranges among populations. The analysis showed significant differences (One-way ANOVA, [F 7, 72] = 15.993, p <0.001). L. melanogaster displayed the narrowest range of thermal tolerance, which differed from all other populations (Scheffe, p <0.05) but not from L. sulphurophila (Scheffe, p = 1.000), L. zonata (Scheffe, p = 0.994) and L. versicolor (Scheffe, p = 0.117). Our data suggested that the most tolerant species to extreme temperatures were L. perugiae, L. yaguajali, and L. vittata (species distributed in low elevations) followed by L. versicolor and L. dominicensis (species which distribution extends into much higher elevations) since these two groups of species displayed broader ranges of temperature tolerance (Fig. 3) and their ranges did not differ significantly from each other (Scheffe, p> 0.05).
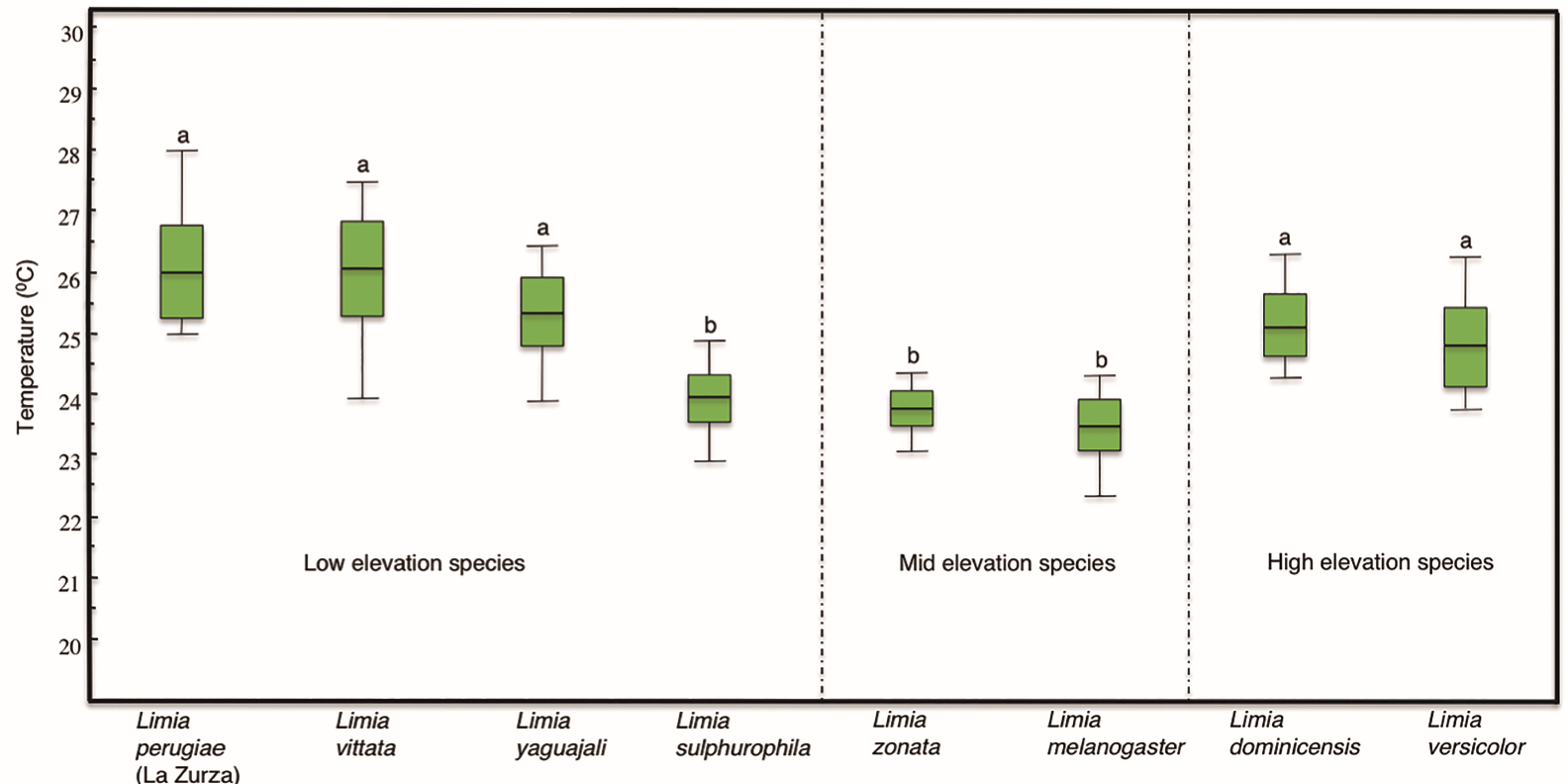
Figure 3. Box plots of the thermal ranges of the eight species of Limia included in this study. Species are grouped according to their elevation distribution in three groups: low elevation species (left), mid elevation species (center) and high elevation species (right). Each box plot represents the median, interquartile ranges, maximum and minimum values of either CTmin or CTmax for each species.
Intra-specific analysis. Population analyses for L. perugiae showed significant differences in CTmax (One-way ANOVA, F [3, 36] = 6.118, p = 0.02) and CTmin (One-way ANOVA, F [3, 36] = 20.982, p <0.001). The population from Lake Enriquillo differed from the other three in both CTmax (Scheffe, p <0.05) and CTmin (Scheffe, p <0.001), (Fig. 4). There were also significant differences in ranges of thermal tolerance among populations of L.perugiae (One-way ANOVA, F [3, 36] = 3.409, p = 0.028). In this case the population from Lake Enriquillo had significant differences with the population from Azufrada (Scheffe, p = 0.037). This is surprising as the Azufrada population is also from Lake Enriquillo, just the north shore, not the south shore.
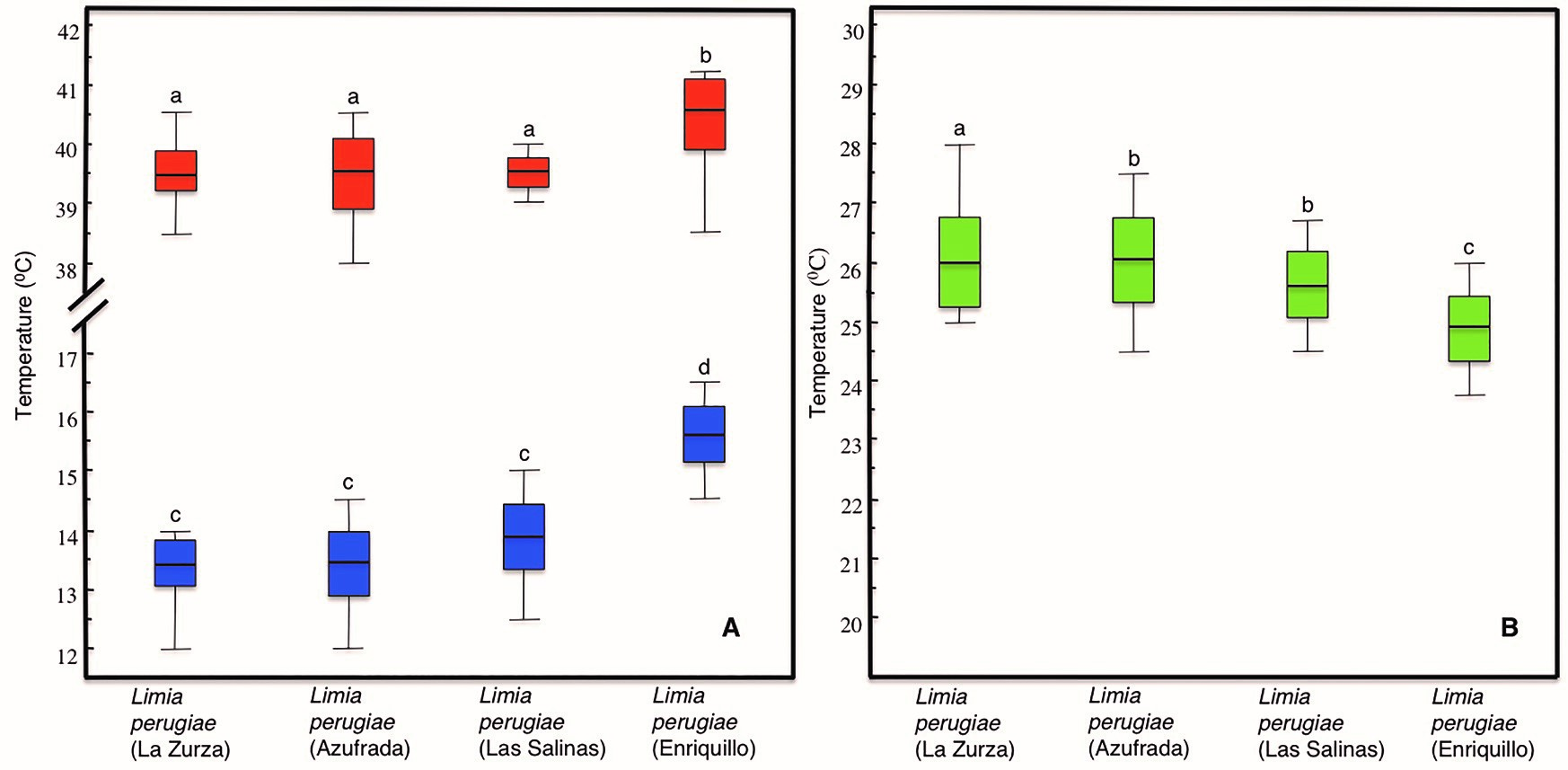
Figure 4. Thermal tolerances of four populations of L. perugiae. CTmin in blue box plots and CTmax in orange box plot (A), and box plots of thermal ranges of the four populations analyzed (B), Each box plot represents the median, interquartile ranges, maximum and minimum values.
DISCUSSION
Our evidence suggests that thermal tolerance and altitudinal distribution of the populations of Limia species analyzed in this study are not related to temperature gradients expected according to Janzen hypothesis. The species studied here showed thermal tolerances not predicted by Janzen’s hypothesis. Generally, even species from high altitudes (for the tropics) have broad thermal tolerances similar to species distributed in low elevations. Hence, the observed pattern does not separate the species as predicted.
Additionally, phylogeny did not explain species relationships according to thermal tolerance. However, failure to detect statistical significance may be due to the rather low number of species included in the analyses. Small sample sizes have been shown to influence the uncertainty and the expected values of most indices of phylogentic signal, including the Pagel’s lambda (λ) (Munkemueller et al., 2012). Powerful phylogenetic comparative analyses typically demand trait and phylogenetic data for over 50 species (Molina-Venegas & Rodriguez, 2017). The genus Limia only has 22 described species (Rodriguez-Silva et al., 2020), which simply cannot satisfy the sampling requirements for robust macroevolutionary inferences.
Temperature tolerance ranges have been shown to shape species distributions and community compositions for some ectotherms in both tropical and temperate climates. Snyder and Weathers (1975) offered experimental evidence on the close relationship between the range of temperature tolerance and the environmental temperature variation in the distribution of several species of amphibians, showing that an increase in the environmental temperature variation also increases the range of temperature tolerance and consequently the distribution range of species. Estimating temperature tolerances and temperature ranges through the analysis of lower (CTmin) and upper (CTmax) thermal limits has been shown to be an efficient and useful method to assess species’ capacity to acclimate to temperature changes in several ectotherms including terrestrial species (i.e. arthropods, reptiles and amphibians) and aquatic organisms (i.e. arthropods, mollusks and fish), (Van Berkum, 1988; Sunday et al., 2011; Buckley & Huey, 2016). However, when using this methodology, the results can be influenced by experimental protocols and conditions in which either CTmin or CTmax are measured. Factors such as acclimation temperature of individuals being tested, the cooling or heating rate used, and the non-lethal endpoint chosen by the experimenter to determine CTmin or CTmax can influence the results (Lutterschmidt & Hutchison, 1997). Fortunately, though, the method has been in use for a long time, which has allowed testing and standardizing protocols for different animal groups. Hence, the technique offers repeatable and rapid quantitative measure of the thermal limits as well as predicts optimal temperate ranges of multiple species (Fischer & Schlupp, 2009; Kingsolver & Umbanhowar, 2018). Although specimens used in this experiment were kept in common garden conditions for different lengths of time, which might influence their tolerance to critical thermal limits; we standardized the acclimation time to a specific temperature range (25 °C - 27 °C) for 45 days under laboratory conditions. This acclimation time is considerably longer than others previously reported in studies of thermal limits in ectothermic organisms (Chanthy et al., 2012; Moyano et al., 2017; Tongnunui & Beamish, 2017), which ensures our data truly reflects the actual thermal tolerances of the species.
Janzen (1967) predicted that species occurring in high altitude in the tropics are specialized for lower temperatures than are low altitude species, which should be better adapted to cope with warmer temperatures. However, our results do not offer support for this prediction suggesting that factors other than temperature shape the distribution in populations of livebearing fishes of the genus Limia. In general, species and populations widespread in lowland habitats (L. perugiae populations from Hispaniola and L. vittata from Cuba) seem to be very tolerant to extreme temperatures (CTmax and CTmin) suggesting that they could also live in mountainous habitats. Given this, what could explain that none of these two species are found in high elevation streams either in Cuba or Hispaniola? We suggest that this is probably due to biotic factors such as competition. In the case of L. vittata in Cuba other dominant livebearing fishes (genera Girardinus and Gambusia) exploit available niches in mountain streams and on Hispaniola other Limia species and also species of Poecilia (P. dominicensis, P. hispaniolana and P. elegans) seem to be restricting L. perugiae to lowland environments. Evolutionary trade-offs between broad tolerance and competitive habitats have been shown to be common in different taxa. For instance, Robinson and Terborgh (1995) showed that interspecific aggression more that habitat suitability might explain spatial segregation patterns observed in Amazonian birds, and Griffis and Jaeger (1998) defined interspecific competition as cause of extinction of a species of salamander (Plethodon shenandoah) in the mountains of Shenandoah National Park, Virginia, USA.
Conversely, among species with distribution ranges that mostly include mid-elevations, there is a less consistent pattern in tolerance to extreme temperatures but with a general trend towards lower ranges of tolerance. Such are the cases of L. melanogaster from Jamaica and L. zonata from Hispaniola, which showed low tolerance ranges and were particularly sensitive to low temperatures. Our recent exploratory work in the Caribbean has recorded these two species associated with permanent freshwater springs and spring runs that buffer temperature fluctuations. The two species abovementioned seem to be physiologically adapted to relatively narrow fluctuations in temperature, which may explain their limited tolerance range.
Another result of our study that runs counter to Janzen’s hypothesis is that two strictly mountainous and locally distributed populations of the species L. versicolor and L. dominicensis, exhibited relatively broad tolerance ranges similar to species that typically are widespread distributed in low elevation environments. Increasing altitude is always accompanied by a decrease in annual average temperature (Sarmiento, 1986), which may indicate high elevation organisms are better adapted to cope with low temperatures. This general pattern was also present in our analysis where the highest study site, in this case Puerto Escondido with one population of Limia dominicensis, exhibited the lowest climate overlap index with the rest of the other sites. However, in tropical ecosystems species living at high elevations might benefit from evolving broad thermal tolerance to deal with diurnal changes in temperature (Ghalambor et al., 2006), which in turn might explain why the two species also show a moderate tolerance for high temperatures. Temperature tolerances of high and mid elevation populations of Limia species relate to results of other studies in tropical amphibians (Navas, 1996; Navas et al., 2013), which showed that species occurring in intermediate elevations were likely stenothermic given the relative thermal stability of those habitats. Conversely, species from higher elevations seemed to have evolved to lead with more changeable temperature (differences between day and night temperatures) and consequently develop broader tolerance ranges.
Our results also provide insights into local physiological adaptations of thermal tolerance within species, which suggests that conspecific populations in diverse habitats have somewhat independent evolutionary pathways (Snyder & Weathers, 1975). First, L. perugiae from the south shore of Lake Enriquillo, near where L. sulphurophila is found, differed from other three populations by showing a narrower thermal breadth. This result together with the lack of phylogenetic signal in thermal tolerance for species emphasizes the importance of biogeographical processes more than just phylogenetic patterns in analyses of climatic niche (Coelho et al., 2019). Second, L. sulphurophila, a locally distributed species mainly known from sulfur springs on the southeastern shore of Lake Enriquillo in the Dominican Republic (Rivas, 1980), seems to be locally adapted to live in a high temperature sulfidic environment and is also physiologically adapted to resist high temperatures. This may prevent competitive exclusion as shown in fish species from temperate climates (Ohlberger et al., 2008).
Our study has implications for conservation and is also pertinent in the context of climate change and species resilience to short-term temperature spikes. Our data provide evidence of species and populations that would be more vulnerable to temperature variation. In this case, the ones occurring in cool permanent freshwater springs in mid elevations and L. sulphurophila (a local endemic species) seemed to be more susceptible to temperature fluctuations because of their narrower thermal breadth. Another issue that may affect conservation of native Limia species is the introduction of invasive livebearing fishes, such as Poecilia reticulata (Guppy). This species has recently been reported as one of the most tolerant ornamental fish to extreme temperatures (Yanar et al., 2019), which may be additional evidence of the invasive success of guppies and in some extend explain why this species becoming dominant in tropical ecosystems.
The implications of temperature for fish physiology and fitness (Niehaus et al., 2012; Payne et al., 2016) make the analysis of thermal limits particularly important in determining distribution of fishes (Culumber et al., 2012). Even though our study does not provide a comprehensive test for Janzen’s hypothesis, it presents evidence at local scales to analyze how elevational gradients may affect the distribution of freshwater fishes, which is a barely studied zoological group in the Caribbean. While it does not offer evidence supporting Janzen’s predictions about climatic variation across elevations, physiological adaptation and species distribution for this group of fish; the study emphasizes the importance of testing the validity of Janzen’s mountain passes hypothesis across multiple taxa. In addition, like previous studies this work stresses the significance of other factors such as species interactions, diet specializations, and even thermoregulatory behavior [as shown by Muñoz % Bodensteiner (2019) in
Caribbean anoles] when interpreting current altitudinal distribution patterns of species.
ACKNOWLEDGEMENTS
This study was supported by the National Geographic Society (WW-054R-17) and the University of Oklahoma. We would like to thank the governments and corresponding ministries of Jamaica and the Dominican Republic for kindly issuing colleting permits. In addition, we thank to the Museo Nacional de Historia Natural “Prof. Eugenio de Jesus Marcano” in Santo Domingo, Dominican Republic and especially to Patricia Torres Pineda and Carlos Suriel. We also thank Carlos Rodriguez from the Ministerio de Educación Superior, Ciencia y Tecnología in the Dominican Republic for his support. Thanks to Ricardo Betancur and Emanuell Ribero their insights and for helping with the analysis of phylogenetic signal. Thanks to Caryn Vaughn, Laura Stein and Bruce Hoagland for their advice and comments on the manuscript. We are grateful to Carolyn Burt, Kerri-Ann Bennett, Stephan Bräger, Heriberto Encarnación Lara, Kenia Ng Alvarado, and Marcos José Rodriguez for help with fieldwork. Trai Spikes, Sophie Huebler, Margaret Zwick, Zeeshawn Beg, and Nabiha Ahmad helped with fish care. Gabriel Costa helped with statistical analysis. Finally, we are grateful to the reviewers for constructive comments on the manuscript.
Literatura Citada
Arnaudo, M. E., N. Toledo, L. Soibelzonand, & P. Bona. 2019. Phylogenetic signal analysis in the basicranium of Ursidae (Carnivora, Mammalia). PeerJ 7:e6597. https:// doi.org/10.7717/peerj.6597
Beitinger, T. L., W. A. Bennett, & R. W. McCauley. 2000. Temperature tolerances of North American freshwater fishes exposed to dynamic changes in temperature. Environmental Biology of Fishes, 58: 237-275.
Buckley, L. B., & R. B. Huey. 2016. Temperature extremes: geographic patterns, recent changes, and implications for organismal vulnerabilities. Global Change Biology, 22: 3829-3842. https://doi:10.1111/gcb.13313
Burgess, G. H., & R. Franz. 1989. Zoogeography of the Antillean freshwater fish fauna. In: Woods, C. A. & F. E. Sergile (Eds) Biogeography of the West Indies: Patterns and Perspectives. CRF Press, Boca Raton FL, 263-304.
Carvajal-Quintero, J. D., F. Escobar, F. Alvarado, F. A. Villa-Navarro, U. Jaramillo-Villa, & J. A. Maldonado-Ocampo. 2015. Variation in freshwater fish assemblages along a regional elevation gradient in the northern Andes, Colombia. Ecology and Evolution, 5 (13): 2608-2620.
Chanthy, P., R. J. Martin, R. V. Gunning, & N. R. Andrew. 2012. The effects of thermal acclimation on lethal temperatures and critical thermal limits in the green vegetable bug, Nezara viridula (L.) (Hemiptera: Pentatomidae). Frontiers in Physiology, 3: 465. https://doi:10.3389/fphys.2012.00465
Coelho, M. T. P., J. F. B Rodrigues, J. A. F. Diniz-Filho, & T. F. Rangel. 2019. Biogeographical history constrains climatic niche diversification without adaptive forces driving evolution. Journal of Biogeography, 46: 1020-1028. https://doi:10.1111/jbi.13553
Cowles, R. B., & C. M. Bogert. 1944. A preliminary study of the thermal requirements of desert reptiles. Bulletin of the American Museum of Natural History, 83: 265-296.
Culumber, Z. W., D. B. Shepard, S. W. Coleman, G. G. Rosenthal, & M. Tobler. 2012. Physiological adaptation along environmental gradients and replicated hybrid zone structure in swordtails (Teleostei: Xiphophorus). Journal of Evolutionary Biology, 25: 1800-1814.
Fischer, C., & I. Schlupp. 2009. Differences in thermal tolerance in coexisting sexual and asexual mollies (Poecilia, Poeciliidae, Teleostei). Journal of Fish Biology, 74: 1662-1668. https://doi:10.1111/j.1095-8649.2009.02214.x
Ghalambor, C. K., R. B. Huey, P. R. Martin, J. J. Tewksbury, & G. Wang. 2006. Are mountain passes higher in the tropics? Janzen’s hypothesis revisited. Integrative and Comparative Biology, 16 (1): 5-17. https://doi.org/10.1093/icb/icj003
Gilbert, P. S., J. Wu, M. W. Simon, J. S. Sinsheimer, & M. E. Alfaro. 2018. Filtering nucleotide sites by phylogenetic signal to noise ratio increases confidence in the Neoaves phylogeny generated from ultraconserved elements. Molecular Phylogenetics and Evolution, 126: 116-128. https://doi:10.1016/j.ympev.2018.03.033
Gingras, B., E. Mohandesan, D. Boko, & W. F. Tecumseh. 2013. Phylogenetic signal in the acoustic parameters of the advertisement calls of four clades of anurans. BMC Evolutionary Biology, 13: 134. https://doi.org/10.1186/1471-2148-13-134
Graham, C. H, A. C. Carnaval, C. D. Cadena, K. R. Zamudio, T. E. Roberts, & J. L. Parra. 2014. The origin and maintenance of montane diversity: integrating evolutionary and ecological processes. Ecography, 37: 711-719. https://doi.org/10.1111/ecog.00578
Griffis, M. R., & R. G. Jaeger. 1998. Competition leads to an extinction-prone species of salamander: interspecific territoriality in a metapopulation. Ecology, 79: 2494-2502.
Hamilton, A. 2001. Phylogeny of Limia (Teleostei: Poeciliidae) based on NADH dehydrogenase subunit 2 sequences. Molecular Phylogenetics and Evolution, 19 (2): 277-289. https://doi:10.1006/mpev.2000.0919
Hijmans, R. J. 2020. Raster: Geographic Data Analysis and Modeling. R package version 3.0-12. https://CRAN.R-project.org/package=raster (accessed: 06/20/2020).
Hua, X. 2016. The impact of seasonality on niche breadth, distribution range and species richness: a theoretical exploration of Janzen’s hypothesis. Proceedings Biological Sciences,
283 (1835): 20160349. https://doi.org/10.1098/rspb.2016.0349
Janzen, D. H. 1967. Why mountain passes are higher in the tropics. American Naturalist, 101: 233-247. https://doi.org/10.1086/282487
Jaramillo-Villa, U., J. A. Maldonado-Ocampo, & F. Escobar. 2010. Altitudinal variation in fish assemblage diversity in streams of the central Andes of Colombia. Journal of Fish Biology, 76: 2401-2417. https://doi.org/10.1111/j.1095-8649.2010.02629.x
Kamilar, J. M., & N. Cooper. 2013. Phylogenetic signal in primate behaviour, ecology and life history. Philosophical Transactions of the Royal Society of London Biological Sciences, 368: 20120341. http://dx.doi.org/10.1098/rstb.2012.0341
Kingsolver, J. G., & J. Umbanhowar. 2018. The analysis and interpretation of critical temperatures. Journal of Experimental Biology, 2018 (221). https://jeb167858. doi:10.1242/jeb.167858
Layne, J. R. Jr., & D. L. Claussen. 1982. The time courses of CTMax and CTMin acclimation in the salamander Desmognathus fuscus. Journal of Thermal Biology, 7 (3): 139-141.
Leiva, F. P., P. Calosi, & W. C. E. P. Verberk. 2019. Scaling of thermal tolerance with body mass and genome size in ectotherms: a comparison between water-and air-breathers. Philosophical Transactions of the Royal Society of London Biological Sciences, 374: 20190035. http://dx.doi.org/10.1098/rstb.2019.0035
Lowe, C. H., & V. J. Vance. 1955. Acclimation of the critical thermal maximum of the reptile Urosaurus ornatus. Science, 122: 73-74.
Lutterschmidt, W. I., & V. H. Hutchison. 1997. The critical thermal maximum: data to support the onset of muscle spasm as the definitive end point. Canadian Journal of Zoology, 75: 1553-1560.
McCain, C. M. 2009. Vertebrate range sizes indicate that mountains may be ‘higher’ in the tropics. Ecology Letters, 12: 550-560. https://doi.org/10.1111/j.14610248.2009.01308.x
Molina-Venegas, R., & M. A. Rodriguez. 2017. Revisiting phylogenetic signal; strong or negligible impacts of polytomies and branch length information? BMC Evolutionary Biology, 17 (53): 1-10.
Moyano, M., C. Candebat, Y. Ruhbaum, S. Alvarez-Fernandez, G. Claireaux, J. L. ZamboninoInfante, & M. A. Peck. 2017. Effects of warming rate, acclimation temperature and ontogeny on the critical thermal maximum of temperate marine fish larvae. Plos One, 12 (7): e0179928. https://doi.org/10.1371/journal.pone.0179928
Munkemueller, T., S. Lavergne, B. Bzeznik, S. Dray, T. Jombart, K. Schiffers, & W. Thuiller. 2012. How to measure and test phylogenetic signal. Methods in Ecology and Evolution, 3: 743-756. https://doi:10.1111/j.2041-210X.2012.00196.x
Muñoz, M. M., J. E. Wegener, & A. C. Algar. 2014. Untangling intra- and interspecific effects on body size clines reveals divergent processes structuring convergent patterns in Anolis lizards. American Naturalist, 184: 636-646.
Muñoz, M. M., & B. L. Bodensteiner. 2019. Janzen’s hypothesis meets the Bogert effect: Connecting climate variation, thermoregulatory behavior and rates of physiological evolution. Integrative Organismal Biology, 1-12. https://doi:10.1093/iob/oby002
Navas, C. A. 1996. Implications of microhabitat selection and patterns of activity on the thermal ecology of high elevation neotropical anurans. Oecologia, 108: 617-626. https://doi.org/10.1007/BF00329034
Navas, C. A., J. M. Carvajalino‐Fernández, L. P. Saboyá‐Acosta, L. A. Rueda‐Solano, & M. A. Carvajalino‐Fernández. 2013. The body temperature of active amphibians along a tropical elevation gradient: Patterns of mean and variance and inference from environmental data. Functional Ecology, 27: 1145-1154. https://doi.org/10.1111/1365-2435.12106
Niehaus, A. C., M. J. Jr. Angilletta, M. W. Sears, C. E. Franklin, & and R. S. Wilson. 2012. Predicting the physiological performance of ectotherms in fluctuating thermal environments. Journal of Experimental Biology, 215: 694-701. https://doi:10.1242/jeb.058032
Ohlberger, J., T. Mehner, G. Staaks, & F. Hölker. 2008. Temperature‐related physiological adaptations promote ecological divergence in a sympatric species pair of temperate freshwater fish, Coregonus spp. Functional Ecology, 22: 501-508.
Pagel, M. 1994. Detecting correlated evolution on phylogenies: a general method for the comparative analysis of discrete characters. Proceedings of the Royal Society of London Biological Sciences, 255: 37-45. https://doi:10.1098/rspb.1994.0006
Payne, N. L., J. A. Smith, D. E. van der Meulen, M. D. Taylor, Y. Y. Watanabe, A. Takahashi, T. A. Marzullo, C. A. Gray, G. Cadiou, & I. M. Suthers. 2016. Temperature dependence of fish performance in the wild: links with species biogeography and physiological thermal tolerance. Functional Ecology, 30 (6): 903-912. https://doi.org/10.1111/1365-2435.12618
Pintanel, P., M. Tejedo, S. R. Ron, G. A. Llorente, & A. Merino-Viteri. 2019. Elevational and microclimatic drivers of thermal tolerance in Andean Pristimantis frogs. Journal of Biogeography, 46: 1664-1675. https://doi.org/10.1111/jbi.13596
Polato, N. R., B. A. Gill, A. A. Shah, M. M. Gray, K. L. Casner, A. Barthelet, & K. R. Zamudio. 2018. Narrow thermal tolerance and low dispersal drive higher speciation in tropical mountains. Proceedings of the National Academy of Sciences, 115: 12471-12476. https://doi.org/10.1073/pnas.1809326115
R Core Team. 2013. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/ .
Revell, L. J. 2012. Phytools: an R package for phylogenetic comparative biology (and other things). Methods in Ecology and Evolution, 3: 217-222. https://doi:10.1111/j.2041-210X .2011.00169.x
Rivas, L. R. 1980. Eight new species of poeciliid fishes of the genus Limia from Hispaniola. Northeast Gulf Science, 4 (1): 28-38.
Robinson, S. K., & J. Terborgh. 1995. Interspecific aggression and habitat selection by Amazonian birds. Journal of Animal Ecology, 64: 1-11.
Rodríguez, C. M. 1997. Phylogenetic analysis of the tribe Poeciliini (Cyprinodontiformes:
Poeciliidae). Copeia, 1997 (4): 663-679.
Rodriguez-Silva, R., P. Torres-Pineda, & J. Josaphat. 2020. Limia mandibularis, a new livebearing fish (Cyprinodontiformes: Poeciliidae) from Lake Miragoane, Haiti. Zootaxa, 4768 (3): 395-404. https://doi.org/10.11646/zootaxa.4768.3.6
Rodriguez, R. S., P. Torres-Pineda, C. M. Rodriguez, & I. Schlupp. 2020. Distribution range extension of Yaguajal Limia, Limia yaguajali (Teleostei: Poeciliidae) from north of the Dominican Republic, Hispaniola. Novitates Caribaea, 15: 127-133.
Sarmiento, G. 1986. Ecological features of climate in high tropical mountains. (11-46). In: Vuilleumier, F., & M. Monasterio (Eds.). High altitude tropical biogeography. Oxford University Press, New York, 649 pp.
Snyder, G. K., & W. W. Weathers. 1975. Temperature adaptations in amphibians. American Naturalist, 109: 93-101.
Spotila, J. R. 1972. Role of temperature and water in the ecology of lungless salamanders. Ecological Monographs, 42 (1972): 95-125.
Sunday, J. M., A. E. Bates, & N. K. Dulvy. 2011. Global analysis of thermal tolerance and latitude in ectotherms. Proceedings of Biological Sciences, 278: 1823-1830.
Tongnunui, S, & F. W. H. Beamish. 2017. Critical thermal maximum, temperature acclimation and climate effects on Thai freshwater fishes. Environment Asia, 10 (1): 109-117.
Valdivieso, D., & J. R. Tamsitt. 1974. Thermal relationships of the neo- tropical frog Hyla labialis (Anura: Hylidae). Life Sciences Occasional Papers Royal Ontario Museum, 26: 1-10.
Van Berkum, F. H. 1988. Latitudinal patterns of the thermal sensitivity of sprint speed in lizards. American Naturalist, 132: 327-343.
Weaver, P. F., O. Tello, J. Krieger, A. Marmolejo, K. F. Weaver, J. V. Garcia, & A. Cruz. 2016a. Hypersalinity drives physiological and morphological changes in Limia perugiae (Poeciliidae). Biology Open, 5: 1093-1101.
Weaver, P. F, A. Cruz, S. Johnson, J. Dupin, & K. F. Weaver. 2016b. Colonizing the Caribbean:
biogeography and evolution of livebearing fishes of the genus Limia (Poeciliidae). Journal of Biogeography, 43: 1808-1819. https://doi:10.1111/jbi.12798
Wollenberg, K. C., I. J. Wang, R. E. Glor, & J. B. Losos. 2013. Determinism in the diversification of Hispaniolan trunk-ground anolis (Anolis cybotes species complex). Evolution, 67: 3175-3190. https://doi.org/10.1111/evo.12184
Yanar, M., S. Erdoğan, & M. Kumlu. 2019. Thermal tolerance of thirteen popular ornamental fish species. Aquaculture, 501: 382-386. https://doi.org/10.1016/j.aquaculture.2018.11.041
